Acute Enterocyte Adaptation to Luminal Glucose: a Posttranslational Mechanism for Rapid Apical Recruitment of the Transporter GLUT2
Rizwan M. Chaudhry*, Jeffrey S. Scow, Srivats Madhavan, Judith a. Duenes, Michael G. Sarr
Gastrointestinal and General Surgery, Mayo Clinic, Rochester, MN
Glucose absorption early after a meal increases markedly to levels far greater than possible by the classic glucose transporter sodium-glucose cotransporter 1 (SGLT1). HYPOTHESIS: High luminal concentrations of glucose lead to rapid (within minutes) phenotypic, non-genomic related adaptations by the enterocyte to recruit another transporter, glucose transporter 2 (GLUT2), to increase glucose absorption. AIM: To determine if glucose absorption increases early postprandially by a GLUT2-mediated mechanism. METHODS: With IACUC approval we perfused in vivo 30 cm jejunal segments in Lewis rats (n=36 rats) for 120 min with isoosmolar solutions of low (10 mM) and high (100 mM) glucose concentrations (n=6 rats in each) containing 14C-D-glucose and 3H-L-glucose to quantitate separately carrier-mediated (stereospecific) and passive (non-stereospecific) absorption, respectively. We also evaluated effects of 1 mM phlorizin (SGLT1 inhibitor) and 1 mM phloretin (GLUT2 inhibitor) at low and high glucose concentrations. RESULTS: Total glucose absorption (carrier-mediated and passive absorption) was much less in 10 mM than the 100 mM glucose solutions (mean±SEM, 2.8±2.0 vs 14.8±3.7 µmol/30 cm/min, respectively; n=6 rats in each; p<0.001). Importantly, stereospecific carrier-mediated uptake increased from 1.9±1.3 to 13.9±2.8 µmol/min (p<0.001), while passive (non-stereospecific) uptake increased, as expected by much less, from 0.7±2.0 to 1.3±1.9 µmol/30 cm/min (p<0.001). In separate experiments (n=6 rats each) carrier-mediated glucose absorption in 10 and 100 mM glucose solutions was decreased by phlorizin (SGLT1 inhibitor) from 2.5±0.2 to 0.6±0.1µmol (p<0.02) and 9.4±2.3 to 2.0±0.9 µmol (p<0.01), respectively, and by phloretin (GLUT2 inhibitor) from 2.4±0.3 to 1.7±0.2 µmol (p<0.02) and 10.0±1.2 to 4.4±1.0 µmol/min, respectively (p<0.03) (Fig.1). The relative contribution of GLUT2 to glucose absorption was 27% at 10 mM but more than double, 56%, at 100 mM (p<0.01). SUMMARY: Augmented, carrier-mediated glucose absorption at infusions of 100 mM glucose appears mediated by GLUT2 (SGLT1 Km≈3-6 mM and would be saturated at ≥20 mM glucose solutions). The marked inhibition of glucose absorption at 100 mM glucose by SGLT1 inhibition (phlorizin) implicates SGLT1 activity as being necessary in this GLUT2-mediated process. There is a GLUT2-mediated component of glucose absorption (~27%) even at 10 mM glucose. CONCLUSION: A small amount of GLUT2 is present apically in the enterocyte (constitutively expressed), but when exposed to high luminal concentrations of glucose, the enterocyte changes its phenotype by recruiting GLUT2 apically (within minutes- thus mediated through a non-genomic, posttranslational mechanism) to markedly augment glucose absorption. This early postprandial process represents a form of “acute phenotypic adaptation”.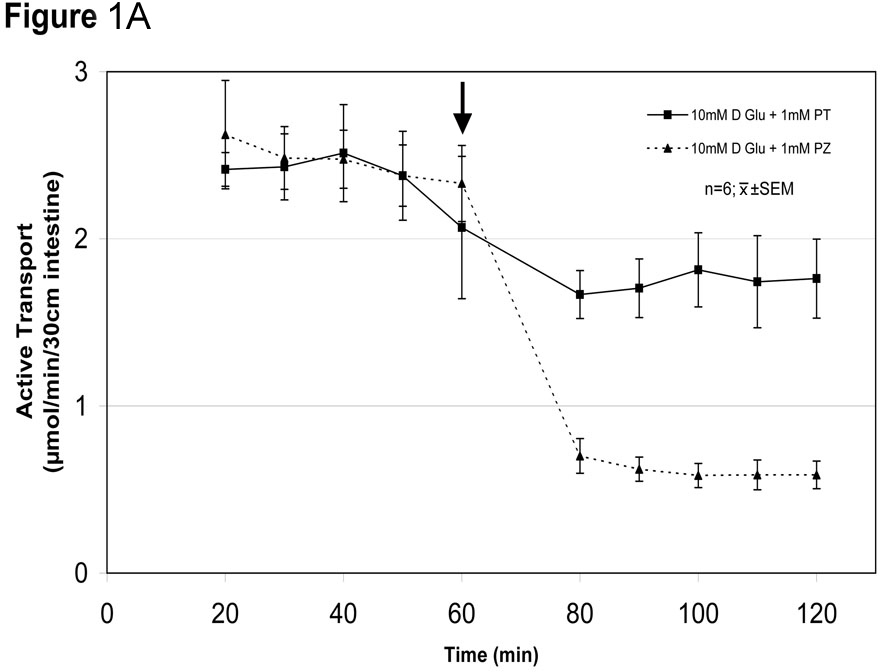
Fig. 1A- Inhibition of in vivo glucose absorption by phloretin (PT) and phlorizin (PZ) at 10 mM glucose (black arrow at 60 min represents administration of inhibitor).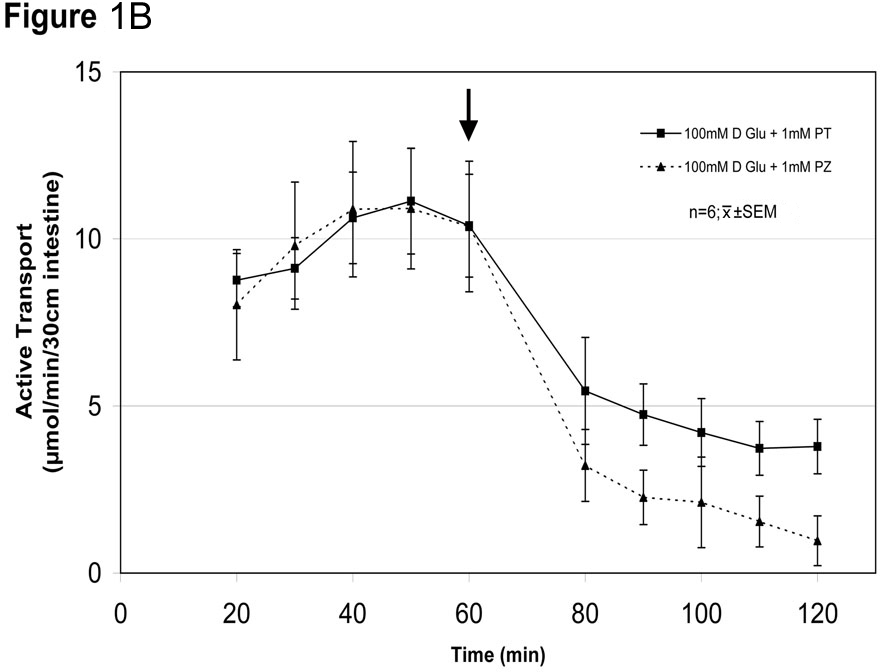
Fig. 1B- Inhibition of in vivo glucose absorption by phloretin (PT) and phlorizin (PZ) at 100 mM glucose (black arrow at 60 min represents administration of inhibitor).
Back to 2011 Program



