POSITIVE HISTOLOGIC MARGINS ARE ASSOCIATED WITH COMPROMISED OVERALL SURVIVAL IN PATIENTS UNDERGOING RESECTION OF INTERMEDIATE AND LARGE GASTROINTESTINAL STROMAL TUMORS
Dhruv J. Patel*1, Joseph N. Fahmy2, Constantine Godellas2, Lawrence Knab2, Gerard Abood2, Frederick Luchette2,3, Marshall S. Baker2,3
1Loyola University Chicago Stritch School of Medicine, Palatine, IL; 2Department of Surgery, Loyola University Medical Center, Maywood, IL; 3Edward Hines, Jr Veterans Administration Hospital, Hines, IL
Background
Current NCCN guidelines recommend that patients undergoing resection of gastrointestinal stromal tumors (GIST) to histologic margins that are microscopically positive (R1) should not, in general, undergo re-resection to clear (R0) margins. Few empiric studies evaluate the impact of histologic margin on overall survival for patients with intermediate and large-sized GIST.
Methods
We queried the NCDB to identify patients undergoing resection for gastric, small bowel, colonic, and rectal GISTs >5cm between 2010 and 2015. Patients with metastatic disease and those receiving neoadjuvant systemic therapy were excluded. Multivariable logistic regression (MVR) was used to identify factors associated with R1 resection. Cox proportional hazard methods were used to evaluate the relationship between R1 resection and overall survival (OS). Patients undergoing resection to R0 margins were then 2:1 propensity score matched for age, sex, Charlson Comorbidity Index (CCI), facility type, insurance status, tumor location, tumor size category (5.1-10CM vs. >10CM), tumor grade (low: '‰¤5 mitoses/50 HPF and high: >5 mitoses/50 HPF), and receipt of adjuvant systemic therapy to those undergoing R1 resection. Kaplan-Meier (KM) analysis was applied to the matched cohorts to assess the association between surgical margin and rates of 5-year OS.
Results
619 patients met inclusion criteria. 349 (56.4%) had gastric GIST, 245 (39.6%) had small bowel GIST and 25 (4.04%) had colorectal GIST. 413 underwent an R0 resection; 206 patients underwent an R1 resection. On MVR, the only factor associated with increased risk of an R1 resection was CCI '‰¥2 (OR 2.17 95% CI [1.01, 4.69]). Use of an MIS approach was not associated with risk of undergoing an R1 resection (OR 1.24 95% CI [0.82, 1.88]). On Cox analysis adjusted for age, demographics, CCI, facility characteristics, surgical approach, tumor location, tumor size, histologic grade, and adjuvant systemic therapy, a positive resection (R1) margin was independently associated with increased risk of mortality (HR: 1.75, CI [1.12-2.76]). Other factors associated with increased adjusted odds risk of mortality included age (HR: 1.04, CI [1.01, 1.07]), small bowel relative to gastric location (HR: 1.63, CI [1.03, 2.58]), tumor size >10CM (HR 1.65, CI [1.02, 2.66], and high histologic grade (HR: 2.29, CI [1.42, 3.69]). Use of adjuvant systemic therapy was not (HR: 0.70, CI [0.42-1.15]) associated with OS. On KM analysis, patients undergoing an R1 resection had a statistically significant reduction in 5-year OS compared to those receiving an R0 resection (69.8% vs 81.4%, p=0.011).
Conclusion
Patients undergoing R1 resection demonstrate compromised OS relative to those undergoing R0 resection of intermediate and large-sized GIST. These results support use of intraoperative efforts to ensure an R0 resection in these patients.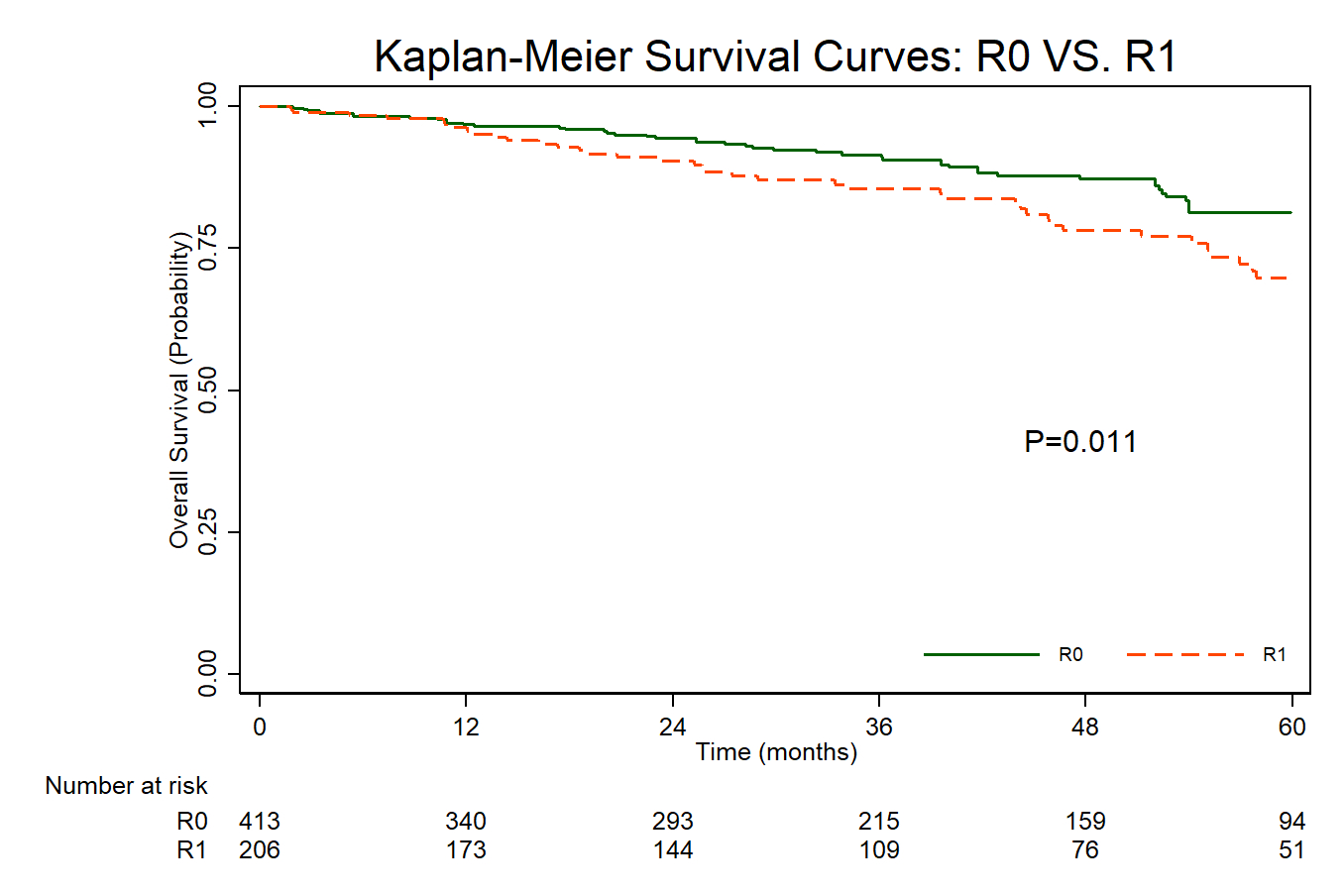
Back to 2021 Abstracts
