LOW RISK, LOW REWARD; A CRITICAL LOOK AT SECRETIN-INDUCED DUODENAL ASPIRATE OF PANCREATIC JUICE (SIDA)
Rachel E. Simpson*1, katelyn flick1, Eugene P. Ceppa1, Michele Yip-Schneider1, Mohammad A. Al-Haddad2, Jeffrey J. Easler2, Stuart Sherman2, John M. DeWitt2, C. Max Schmidt1
1Surgery, Indiana University, Indianapolis, IN; 2Gastroenterology, Indiana University School of Medicine, Indianapolis, IN
Introduction:DNA profiling of pancreatic fluid is commercially available to risk-stratify patients with pancreatic lesions. EUS-FNA of the pancreas holds inherent risk, and not all patients have lesions amenable to sampling. Secretin-Induced Duodenal Aspiration (SIDA) of pancreatic duct fluid has been proposed as an alternate screening test for pancreatic neoplasms to overcome these limitations. Use of SIDA in published literature is mostly focused on high-risk patients (family history/genetic syndrome) or patients with PDAC, with reported mutation detection rates reaching 90% depending on indication. We sought to determine the clinical yield and safety of commercially analyzed SIDA samples in low- and high-risk populations.
Methods:A prospectively maintained institutional database of pancreatic fluid DNA profiles was retrospectively reviewed. Patients with SIDA fluid analysis were identified, as well as prior/concomitant DNA profiles of EUS-FNA pancreatic cyst or main duct fluid. The most common SIDA protocol included aspiration of gastric contents, administration of 16mcg of intravenous secretin followed 10 minutes later by aspiration of duodenal fluid for genetic analysis (Interpace Diagnostics; Parsippany, NJ). DNA quantity/quality, oncogene mutations (KRAS/GNAS), and allelic loss of heterozygosity (LOH) were recorded. Specimens with "No Amplification"? were excluded. Fisher's Exact test was used to compare mutation incidence.
Results:From 2016-2018, 57 patients underwent SIDA testing for intraductal papillary mucinous neoplasms (IPMN) (n=43); undifferentiated solitary cyst (n=9); surveillance for family history of PDAC only (n=3); and pancreatitis (n=2). SIDA mutation yield was low: 1 KRAS (1.9%) and 1 GNAS (2.2%) mutation were detected. One patient displayed allelic LOH (2.3%). Thirty-seven concomitant EUS-FNA samples of pancreatic cyst (n=35) or main duct fluid (n=2) with DNA profiling revealed 12 KRAS (36.4%) and 3 GNAS (9.7%) mutations, but no allelic LOH. No KRAS/GNAS/LOH mutations detected on SIDA were present on EUS-FNA and vice-versa. Compared to EUS-FNA, SIDA samples had a greater incidence of elevated DNA quantity (71%vs 32%; P<0.001) and good DNA quality (47%vs 27%; P=0.081). All patients had a benign outcome based on DNA profile (n=15) or at last follow-up with clinical exam, imaging and/or pathology (n=42, median follow-up 16 months). Three patients experienced post-procedural pancreatitis; each had concomitant EUS-FNA samples at the time of SIDA. No patients with only SIDA experienced a complication.
Conclusion:The diagnostic yield of commercially-analyzed SIDA samples (2%) was much lower than previous reports. These SIDA profiles may not accurately reflect cyst or main pancreatic duct contents. The safety profile of SIDA testing may be better than that of EUS-FNA, but the utility of commercially-analyzed SIDA samples is unclear.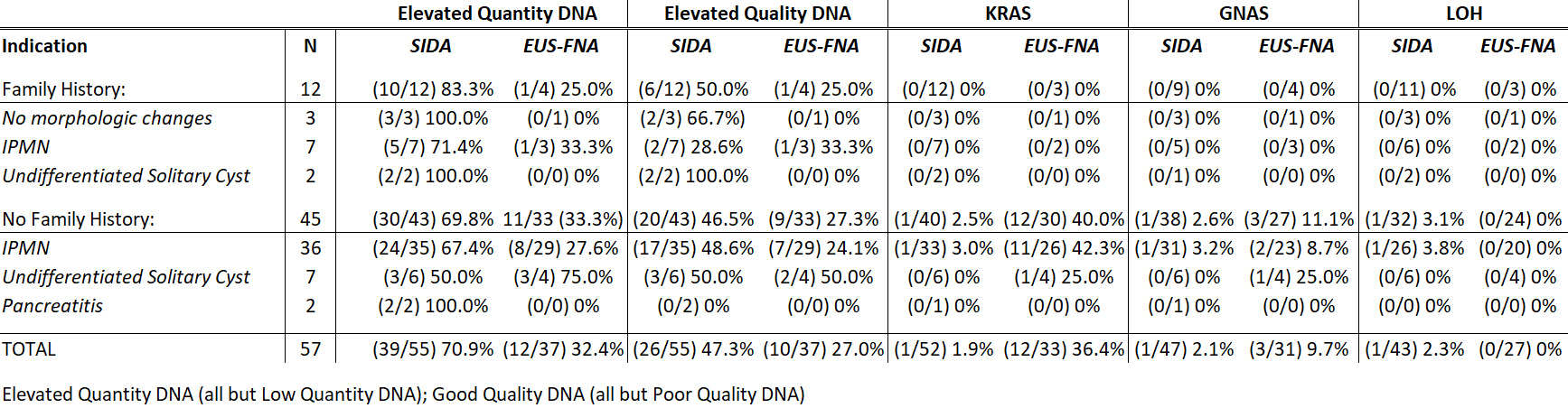
Back to 2019 Posters




