REAL WORLD SCENARIO: CROSS REGIMEN AS PREOPERATIVE THERAPY FOR ESOPHAGEAL SQUAMOUS CELL CARCINOMA
Ian Yu Hong Wong*1,5, Ka On Lam2,4, Wendy Chan2, Claudia Wong1, Tsz Him So2, Kwan Kit Chan1, Cheuk Wai Choi2, Tsz Ting Law1, Keith Chiu3, Siu Y. Chan1,5, Dora Lai Wan Kwong2,4, Simon Law1,5
1Surgery, The University of Hong Kong, Hong Kong, Hong Kong; 2Clinical Oncology, The University of Hong Kong, Hong Kong, Hong Kong; 3Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong; 4Clinical Oncology, University of Hong Kong-Shenzhen Hospital, Shenzhen, China; 5Surgery, University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Background
Preoperative chemoradiation using the CROSS regimen has been widely adopted worldwide since its publication in 2012. Imparted survival advantage is especially impressive for squamous cell carcinoma (SCC). This study aimed at investigating the efficacy of the CROSS regimen in real world scenario in a single high-volume tertiary centre.
Methods
This is a retrospective study of all esophageal SCC patients intended for preoperative chemoradiation using the CROSS regimen during 2012-2017. Data were analysed using a prospectively collected database. Inclusion criteria in CROSS trial included adenocarcinoma and SCC of the thoracic esophagus and cardia, maximal tumour length of 8cm, Clinical stage (AJCC 6th edition) T1N1 or T2-3N0-1 and M0, 18-75 years of age, WHO performance status score of 2 or lower. We divided our cohort into two groups: those patients within the selection criteria in the CROSS trial, and those with more advanced disease. Clinical outcome and survival data were compared.
Results
Eighty-eight patients were given preoperative chemoradiation according to the CROSS protocol. There were 42 patients in the "Within CROSS"? group and 46 in the "Beyond CROSS"? group. Compared to the original published data in the CROSS trial, our patients recruited using the same inclusion criteria ("Within CROSS cohort"?) were older, had longer tumour, more advanced disease by stage and worse performance status numerically (Fig 1). The "Beyond CROSS"? group had more advanced disease. Esophagectomy was performed in 33 (79%) patients in the "Within CROSS"? group and 28 (61%) patients in the "Beyond CROSS"? Group (p=0.123). The median number of lymph nodes harvested were 38 & 35 respectively (p=0.621). Pathological complete remission (pCR) rate was 33% vs 21% respectively (p=0.267). By comparisons, in the original CROSS trial, Esophagectomy was performed in 161 (90%) patients with a median of 15 lymph nodes harvested. The overall pCR rate was 29 % (SCC subgroup, 49%). By intention-to-treat, the median survival of "Within CROSS"? and "Beyond CROSS"? groups were 29 months vs. 14 months, respectively (p=0.132), a trend favouring "Within CROSS"? Group (Fig 2). Survival in the original CROSS trial was much more impressive.
Discussion
The survival benefit of CROSS regimen in esophageal SCC patient was shown to be exceptionally promising in the trial setting. In a real world scenario, using the same inclusion criteria, we encountered patients that were older, with more advanced disease and poorer functional status. Despite adequate surgery and lymph node dissection, outcome remains suboptimal and the excellent results in the trial setting were not repeatable. For patients having more advanced stage than those included in the CROSS trial, the prognosis was even more disappointing. The CROSS regimen may not be adequate. The optimal multimodal therapy program remains uncertain.
Comparison of baseline demographics and outcome between "Overall", "Beyond CROSS", "Within CROSS" and published figures in CROSS Trial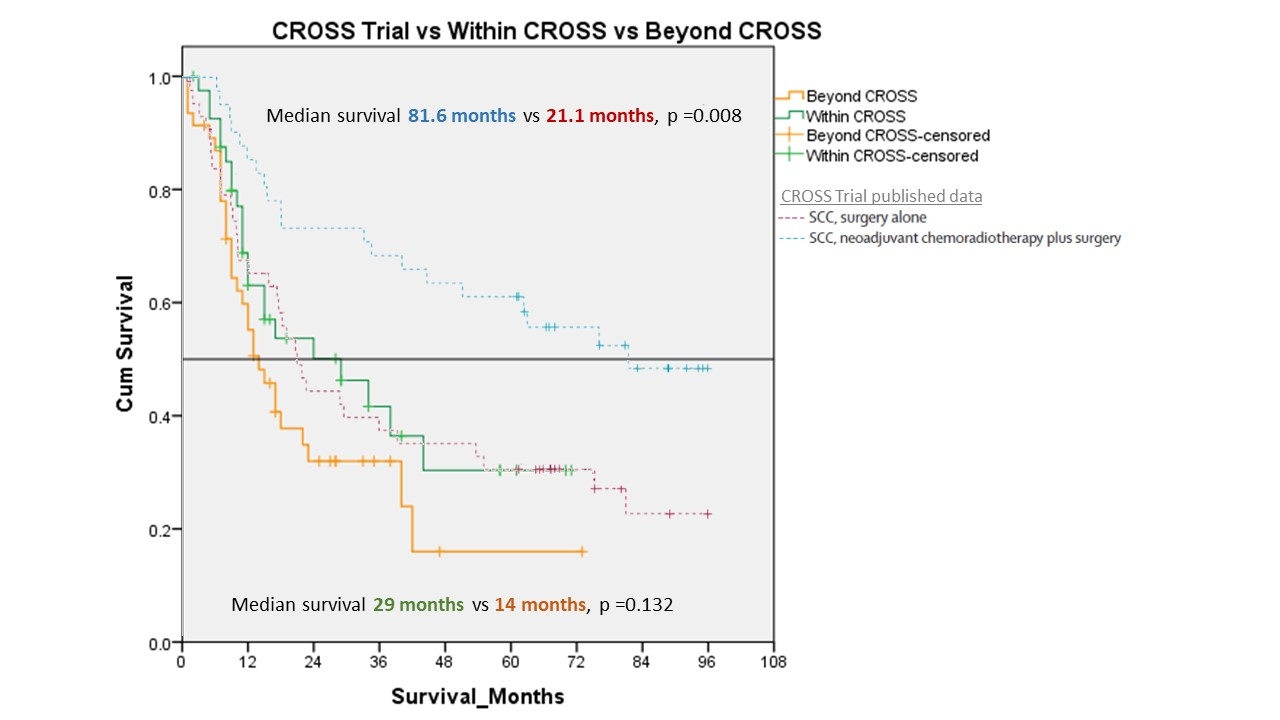
Kaplan Meier survival curve of "Within CROSS" & "Beyond CROSS" group in comparison to published data from CROSS Trial
Back to 2019 Abstracts




