INTRODUCTION –
The Enterra device is implanted for treatment of refractory gastroparesis (diabetes or idiopathic). After implantation an interrogator is used to program and monitor the neurostimulator. For effective communication a specific side of the device needs to be positioned superficially towards the skin. If the device "flips," the non-communicable side will face the interrogation probe and the interrogation device will be unable to communicate with the neurostimulator. Therefore, ensuring that the device is maintained in the proper position is vital for the function of the system. One method to achieve this is by fixing the device to the abdominal fascia in the subcutaneous pocket through pre-placed holes on one side of the device housing. However, it has been observed that this fixation configuration may, in fact, act as a "hinge" promoting flipping. For our study, our hypothesis is that fixation of the device to the abdominal fascia in its subcutaneous pocket will be associated with more episodes of flipping.
METHODS –
A retrospective analysis of 108 patients who underwent Enterra device placement for idiopathic or diabetic gastroparesis between 2012 and 2023 was completed. Eighty-four patients underwent Enterra device placement without fascia fixation, while twenty-four patients had fascial fixation. Records were reviewed for patients' BMI, age, sex, obesity status, type of gastroparesis, fixation of the Enterra device to the fascia, and the length of follow-up. Associations were investigated using the Mann-Whitney U test or Fisher's exact test.
RESULTS –
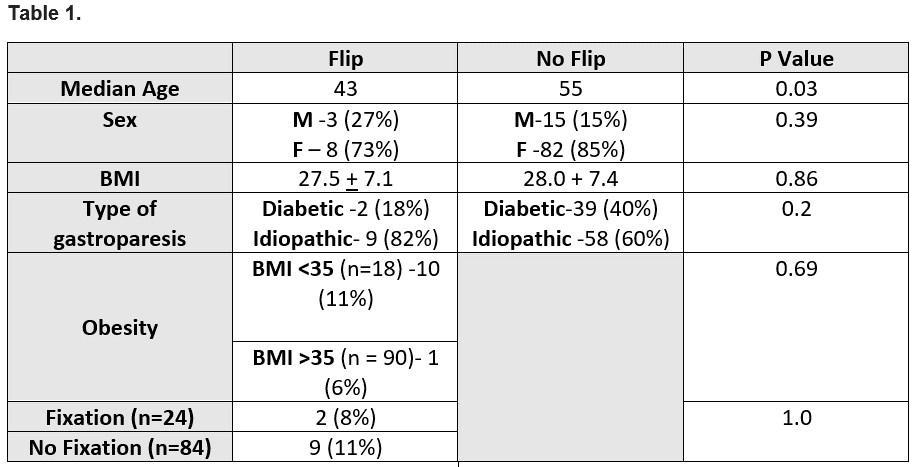
In patients with a flipped device 9 (11%) had no fixation while 2 (8%) did, however this did not reach statistical significance (p 1.0). Follow-up lengths did not differ between groups (1036 days No Flip group vs 1508 days Flip group, p 0.14). Device flipping was not affected by BMI (p 0.69).
Patients who experienced a flipped Enterra device were younger with a median age of 43 years (37.75 – 49.25) than patients who didn't have a flipped Enterra device with a median age of 55 (43 – 63) (p 0.03). Gender did not affect device flipping (p 0.39). There was no differences between the types of gastropareses (idiopathic and diabetic) in term of Enterra device flipping (p 0.2).
CONCLUSION –
Device fixation to the fascia does not appear to have a significant association in the rate of Enterra device flipping. Sex, BMI, obesity, and type of gastroparesis also did not have a relationship to flipping of the Enterra device. However, younger age appears to affect device flipping.