Introduction: Endoscopic full thickness resection (ETFR) using the full thickness resection device (FTRD) allows for transmural resection of lesions in the gastrointestinal tract. FTRD is widely used for removal of non-lifting or previously manipulated polyps. However, there is limited data regarding its efficacy for the removal of early colorectal cancer (CRC). The FTRD involves the use of forceps to grasp the lesion into the cap prior to deployment of a pre-loaded clip followed by subsequent resection. It is hypothesized that due to the superficial invasion nature of some early CRCs, the forceps might not be able to completely pull the lesion into the cap, making R0 resection challenging. Therefore, we aimed to evaluate the feasibility, safety, and efficacy of the FTRD for the removal of early CRC.
Methods: We conducted a multicenter retrospective study to assess patients who underwent EFTR for suspected or biopsy proven colorectal adenocarcinoma. Patients with final histology other than adenocarcinoma were excluded from analysis. Primary outcomes were technical success, defined as achievement of en-bloc endoscopic resection and R0 resection, defined as tumor free lateral and deep resection margins (?0.1 mm) on histopathology. Secondary outcomes were recurrence rate, need for follow up surgical procedure and adverse events.
Results: 15 patients, mean age 68+/-12.18 years, comprising 9 (60%) males were included. Six patients underwent EFTR for optically suspected and nine for biopsy proven adenocarcinoma. The FTRD was used as monotherapy in 12 cases and in conjunction with ESD (hybrid ESD-EFTR: 1 case) and EMR (hybrid EMR-EFTR: 2 cases). Technical success of procedure was 100% (15/15). Ro resection rate was 86.66% (13/15) and full thickness resection rate was 100% (15/15). Table 1 summarizes procedural and pathologic characteristics of lesions. Final histopathology revealed T1 CRC in 10 cases and T2 CRC in 5. Negative margins were achieved in 86.66% (13/15). R0 section was not achieved in a patient with T1 CRC and a patient with T2 CRC each. Post EFTR, surgery was done in 26.66% (4/15) patients due to high risk histological features seen on the resected specimen. Follow up endoscopy was done in 9/11 remaining patients, at mean interval of 20.16+/-17.11 weeks. None of the patients had residual lesion and surveillance biopsy showed scar tissue in 5/5 patients. Overall 4 adverse events occurred which has been summarized in Table 2.
Conclusions: Our study provides the first multicenter experience using the FTRD for resection of CRC from North America. Our preliminary data suggests that the FTRD can be used for resection of early CRC in select cases. Longitudinal data from this ongoing registry should provide valuable information on long term outcomes with the use of FTRD for CRC.
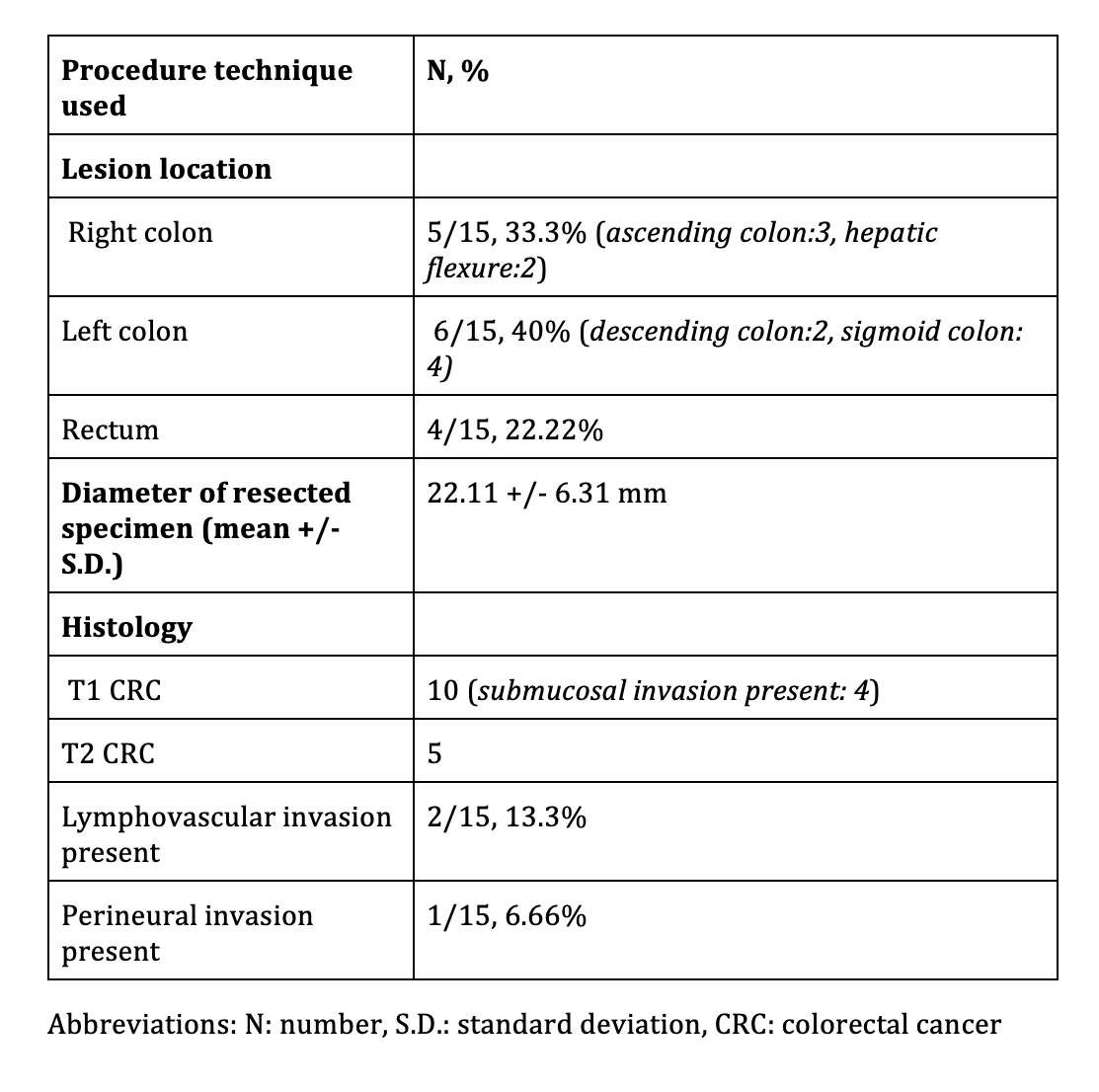
Table 1: Details of location of lesion and histology of resected specimen
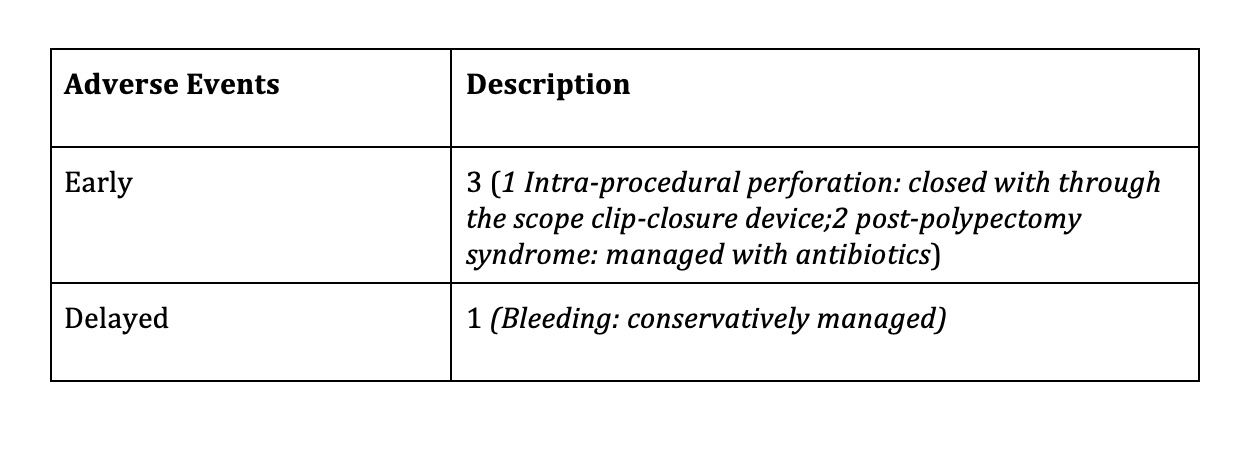
Table 2: Safety outcomes of endoscopic full thickness resection using full thickness resection device in early colorectal cancer