Introduction
The Roux-en-Y gastric bypass (RYGB) is a proven surgical intervention for the treatment of obesity and metabolic disease. Approximately 20-30% of patients regain more than 15% of their body weight 5 years after RYGB. Glucagon-like peptide-1 receptor agonists (GLP1A) are a viable option to treat weight regain following a RYGB. Established side effects of GLP1As include nausea and abdominal pain due to delayed gastric emptying. GLP1A-associated delayed gastric emptying may also promote distension of the excluded stomach and pancreaticobiliary limb in patients after RYGB. Due to the increase in utilization of GLP1A there is a need to identify potential GLP1A treatment associated adverse effects in patients after RYGB. We sought to characterize this phenomenon in a case series of patients at a single academic center in the United States.
Methods
Following a detailed chart review, we identified 40 patients on GLP1A treatment for weight regain after RYGB. In this cohort, we observed a distended excluded stomach and/or dilated biliopancreatic limb on computed tomography (CT) of the abdomen in 4 (10%) patients. We collected baseline demographic data and symptoms at presentation. Abdominal imaging was reviewed with a radiologist to confirm dilation.
Results
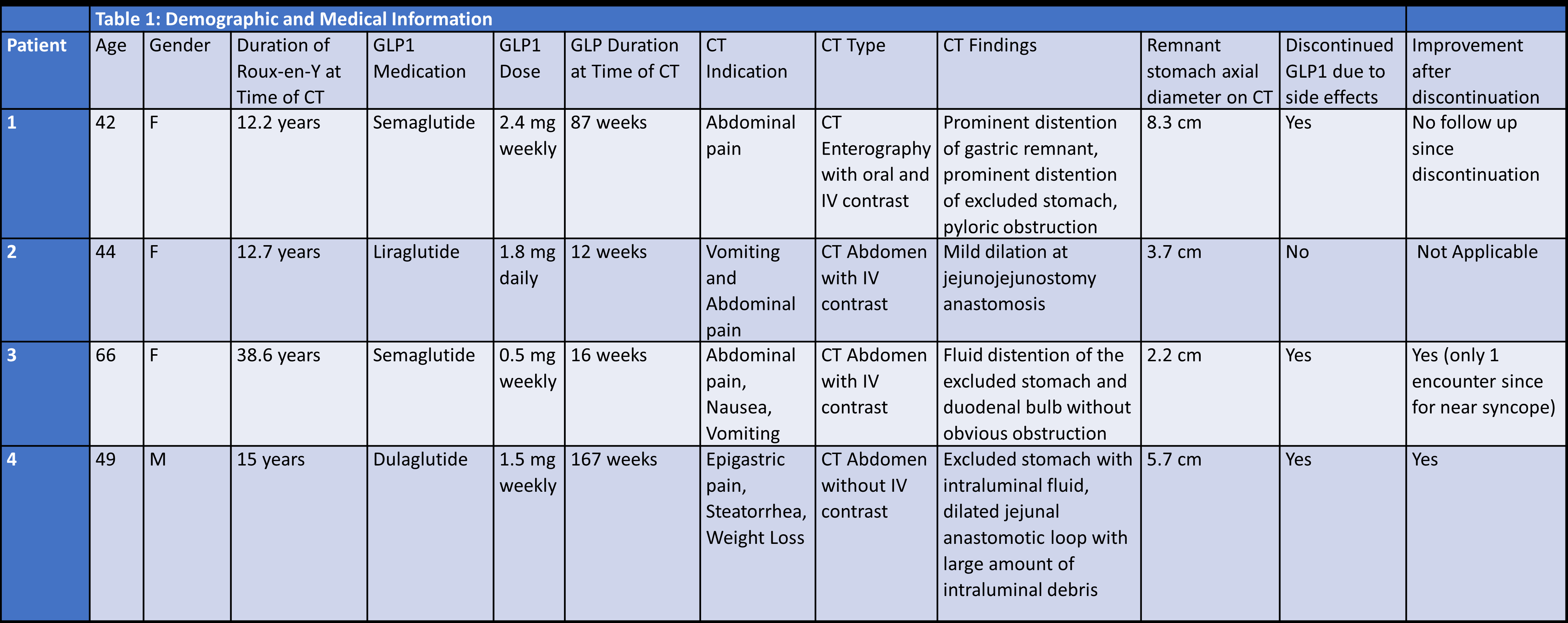
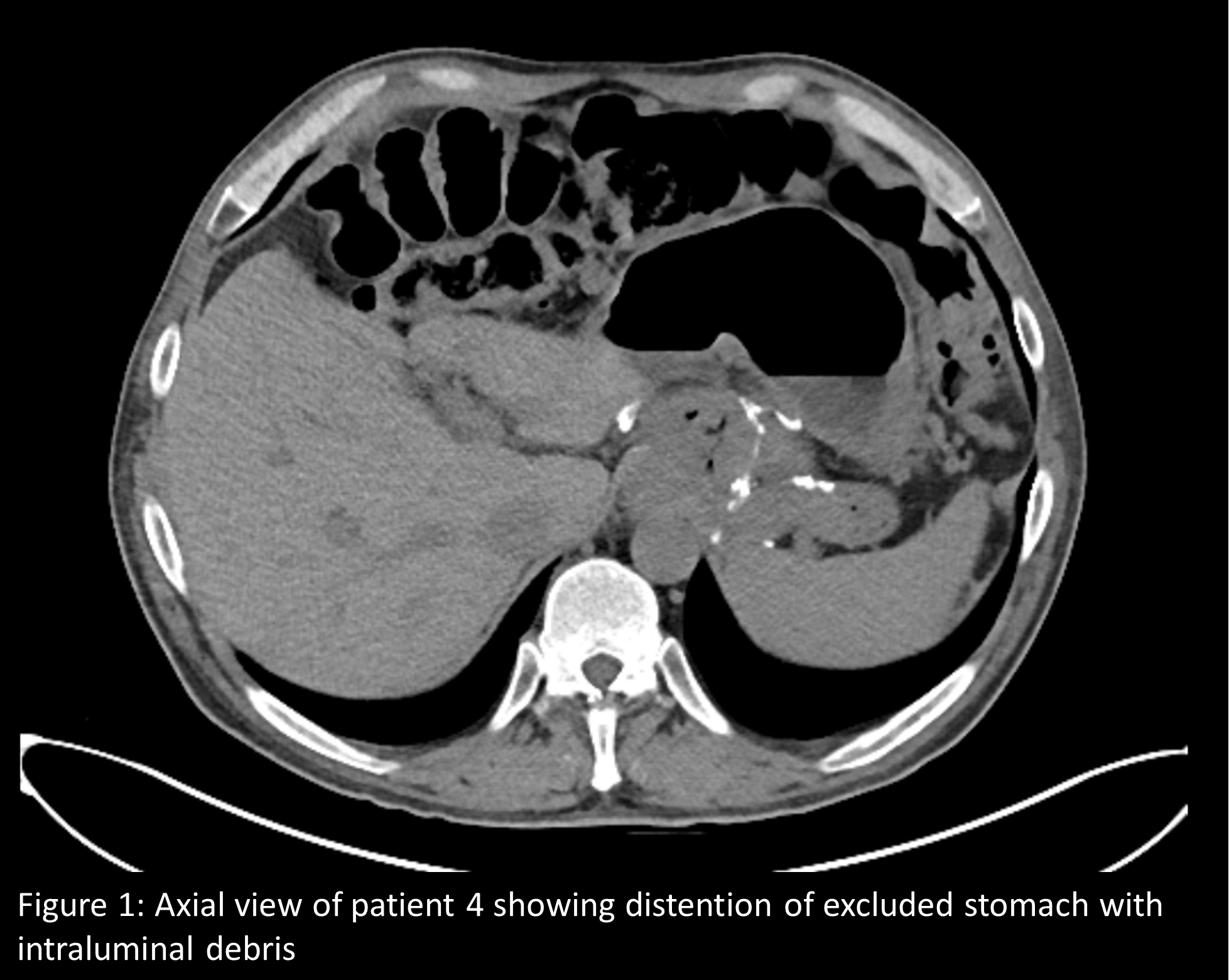
In all 4 patients, CT imaging had been done on account of abdominal symptoms. All four patients were evaluated for abdominal pain and two also presented with nausea and vomiting. Median age was 46.5 years (interquartile range [IQR] 14.5 (Table 1). The duration of RYGB at time of CT ranged from 12.2 to 38.6 years. Two patients were treated with semaglutide, one with liraglutide, and one with dulaglutide (Table 1). Treatment duration with GLP1A at time of CT imaging ranged from 12 weeks to 167 weeks (IQR 113). Different abdominal CT imaging protocols were utilized including intravenous and oral contrast and all four patients exhibited varying segments of distention along the biliopancreatic limb (Table 1). In three out of four patients, GLP1A was discontinued due to concern for its contribution to underlying pain, nausea, and vomiting. Of the three patients who discontinued GLP1A two were noted to have improvement of symptoms.
Conclusion
As GLP1As are increasingly utilized in clinical practice, it is imperative that we characterize potential side effects and complications in special populations like post gastric bypass patients. More research is needed to identify patient-level predictors of excluded stomach and biliopancreatic limb distension among patients on GLP1A after Roux-en-Y gastric bypass.