Back to 2024 Abstracts
THE MICROBIOTA AT THE TIME OF SURGERY IS SIGNIFICANTLY ENRICHED WITH PRO-TUMORIGENIC BACTERIA IN PATIENTS WHO DEVELOP A POSTOPERATIVE RECURRENCE
Meejeon Roh, Mohsen Rouhani Ravari
*, Claire Wild, Mason Vierra, Ryan B. Morgan, Lindsay Alpert, Nicholas R. Suss, Olga Zaborina, Benjamin D. Shogan
University of Chicago Division of the Biological Sciences, Chicago, IL
Introduction: The role of bacteria in the pathogenesis of colorectal cancer (CRC) recurrence following surgery is unknown. Using a mouse model that mimics human CRC recurrence, we have shown that postoperative tumor formation is dependent upon the colonic colonization of bacterial strains that promote a metastatic phenotype in cancer cells. Whether these microbes contribute to human recurrence is unstudied. We hypothesize that the microbiota of humans who develop recurrence after surgery are significantly enriched with pro-tumorigenic bacteria.
Methods: Patients undergoing resection for CRC were eligible, and all subjects received a bowel prep with Golytely, neomycin, and metronidazole. Fecal samples were collected on the day of surgery by recovering intraluminal stool from the resected segment. Migration assays were performed
in vitro by incubating either MC38 or CT26 CRC cells with either whole intraluminal fecal content or individual bacterial strains that were isolated from the fecal sample. Bacterial strains that were identified to promote cancer cell migration were then tested for their effect on tumor formation in an established mouse model of colon surgery.
Results: 19 consecutive patients who underwent surgery for CRC were included. After a median follow-up of 30.4 months, 6 patients (31.6%) developed a recurrence. Patients who developed a recurrence had a nearly 25-fold increase in bacterial load on the day of surgery compared to patients who did not develop a recurrence (16S rRNA copies: 1.9x10
8 vs 8.1x10
6; p=0.01). To study the role of bacteria on CRC recurrence, three age-BMI patients from each cohort were chosen to assess their intraluminal contents on cancer cell migration. The intraluminal contents recovered from patients who developed a recurrence significantly increased the migration of both CT26 and MC38 cells compared to samples from patients who did not develop a recurrence (mean+/-SD CT26: 204+/-35.7 vs 330+/-30.1; p<0.01; MC38: 98+17.4 cells vs 181+/-564.1; p<0.01)(
Fig1). To determine if there was a strain within the whole contents that was driving this migratory phenotype, all culturable strains from each sample were recovered. From patients that developed recurrence, we identified strains of
Pseudomonas aeruginosa, Enterococcus avium, and
Enterococcus durans that significantly enhanced cancer cell migration. Strikingly, when these human-derived pro-migratory strains were introduced into mouse model, all strains significantly promoted postoperative tumor formation compared to control (
Fig2). No pro-migratory strains were recovered from patients who did not develop a recurrence.
Conclusion: The microbiota in patients who develop a postoperative recurrence is enriched with bacteria that can promote tumorigenesis. Preventing colonization of these strains during the perioperative period may decrease postoperative recurrence rates.
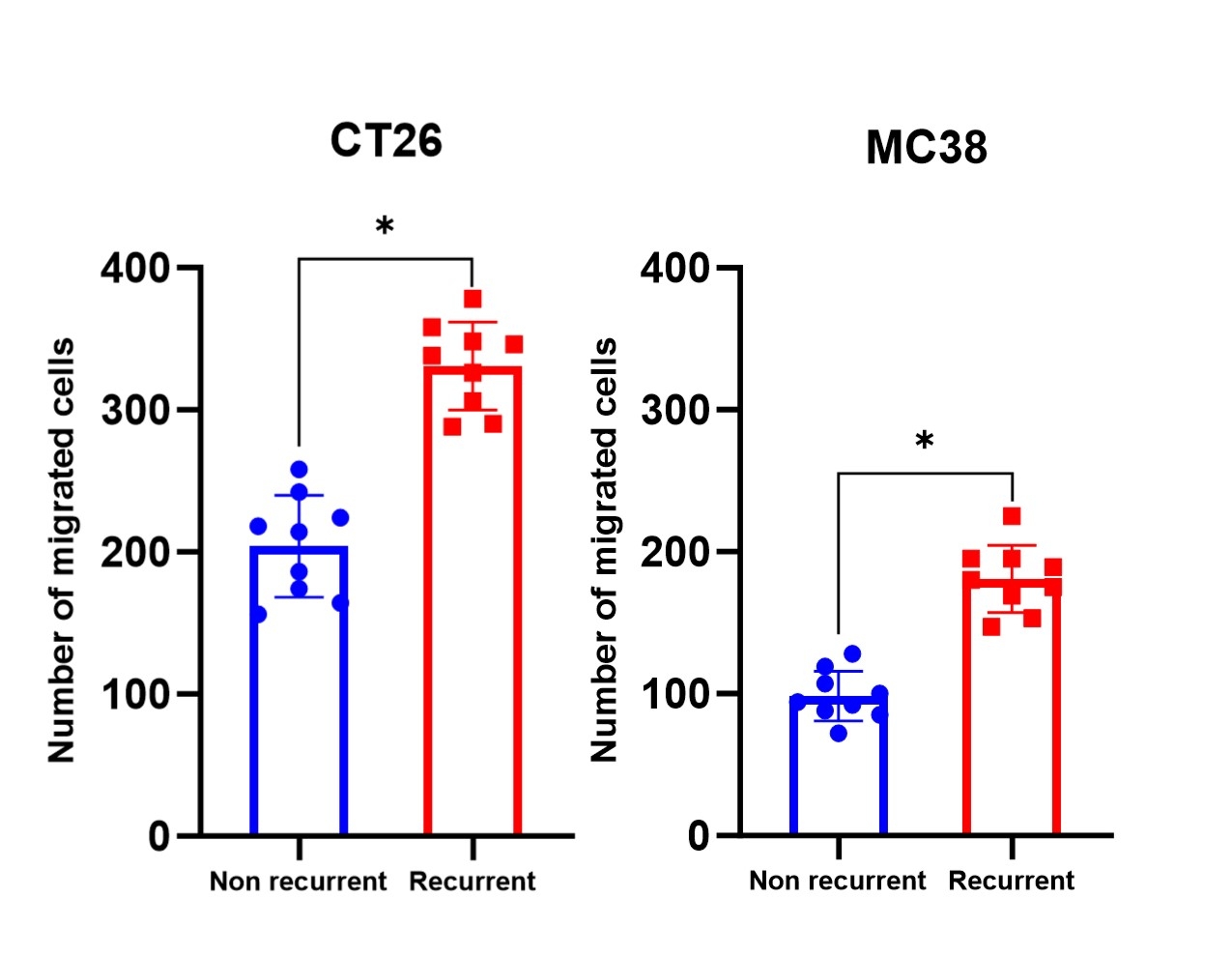 Fig 1.
Fig 1. The intraluminal contents recovered from patients who developed a recurrence significantly increased the migration of both CT26 and MC38 cancer cells compared to contents recovered from patients who did not develop a recurrence. n=3 patents per cohort run in triplicate; *p<0.0001
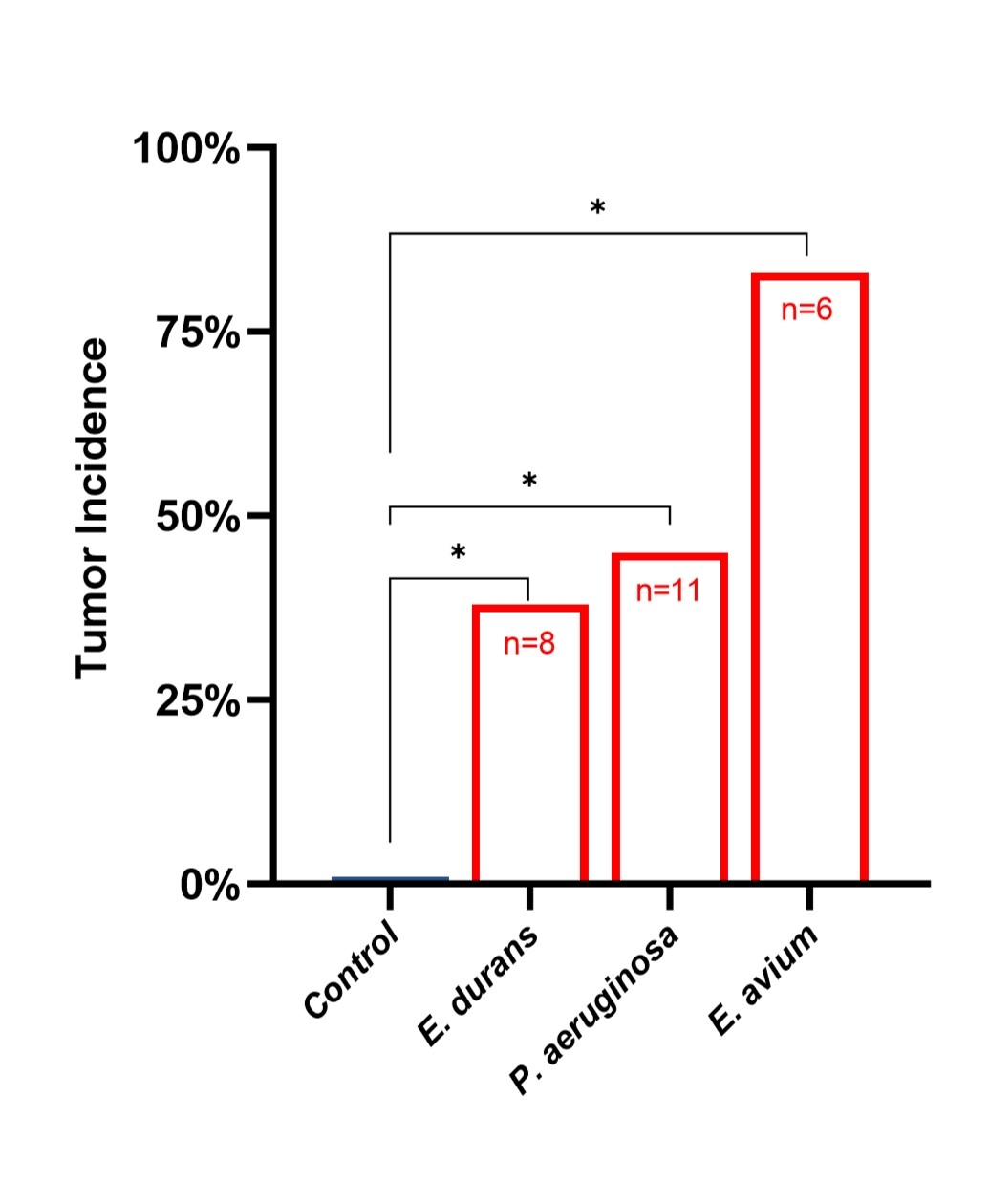 Fig 2.
Fig 2. Postoperative tumor formation in a mouse model of colon surgery is significantly increased by colonic colonization with the pro-migratory strains recovered from human patients who developed recurrence. Mice underwent colonic anastomoses, colonized with the test bacteria on POD1 via enema, exposed to CT26 cancer cells on POD2, and assessed for local anastomotic tumor formation on POD21. *p<0.05
Back to 2024 Abstracts

