Back to 2024 Abstracts
TREATMENT OF RECTAL CANCER BY TOTAL NEOADJUVANT THERAPY AND TRANSANAL LIMITED LOCAL EXCISION FOR COMPLETE RESPONDERS: PHASE II TRIAL RESULTS
Jasneet S. Bhullar
*2, Amer Alame
1, Ernesto Drelichman
2, Mohammed Barawi
1, Amer Zeni
2, Zyad Kafri
1, Elon Knoll
3, Yakan Adli
2, Wajahat Khan
3, Amr Aref
11Ascension St John Hospital, Detroit, MI; 2Ascension Providence Hospital Southfield Campus, Southfield, MI; 3Ascension Macomb-Oakland Hospital Warren Campus, Warren, MI
BACKGROUNDTotal Mesorectal Excision (TME) with or without neoadjuvant/adjuvant therapy is the standard of care treatment for locally advanced rectal cancer; however it can be associated with long term frequent and significant morbidity. Replacing TME by either watchful waiting (WW) or local excision (LE) in a selected group of patients, who respond favorably to neoadjuvant treatment, may improve the post-therapy quality of life without compromising treatment outcomes. Recently there has been reluctance to pursue the LE approach, despite its clear advantage of immediate confirmation of the ypT status of the resected tumor, because of the perceived morbidity associated with this procedure.METHODSPatients were eligible for enrollment on this trial (NCT 03941366) if they were diagnosed with non-metastatic, locally-advanced invasive adenocarcinoma of the rectum with clinical stage T3N0-N+, T2N+ or T2N0 if sphincter preservation surgery without neoadjuvant treatment was not feasible. All patients received 6 cycles of induction FOLFOX followed by concurrent Capecitabine/5FU infusion and external beam radiation. Patients with tumors in complete clinical response after final restaging underwent ‚ÄúLimited full thickness LE‚Ä? (LLE) where the residual mucosal abnormality and the entire overlying rectal wall were resected with minimal or no margin of the surrounding normal tissues. Patients with tumors exhibiting less than complete clinical response were offered TME.RESULTSTwenty-four patients were enrolled in this trial during the period of 2016-2023. All patients completed total neoadjuvant therapy (TNT) as planned. Two patients are still in the observation period after completion of TNT. Table 1 summarizes the clinical parameters of the 22 patients who completed the final restaging. Primary TME was performed in 9 patients because of lack of CCR and 13 patients were triaged to LLE which was performed on only 12 patients as 1 patient was managed by WW. Completion TME was recommended for 2 patients but done only in 1 case because of patient's refusal.Among the 12 patients who had LLE, Five patients were discharged on the same operative day and 7 patients had a 1 day hospital stay. Major complications occurred in only 1/12 patients. Eleven patients were managed solely by LLE and only 1 patient developed local recurrence. To date, 10/22 patients avoided TME and ultimate pelvic control was achieved in all patients.CONCLUSIONOrgan preservation via LLE for complete responders after TNT is not associated with frequent and severe morbidity and results in good local control.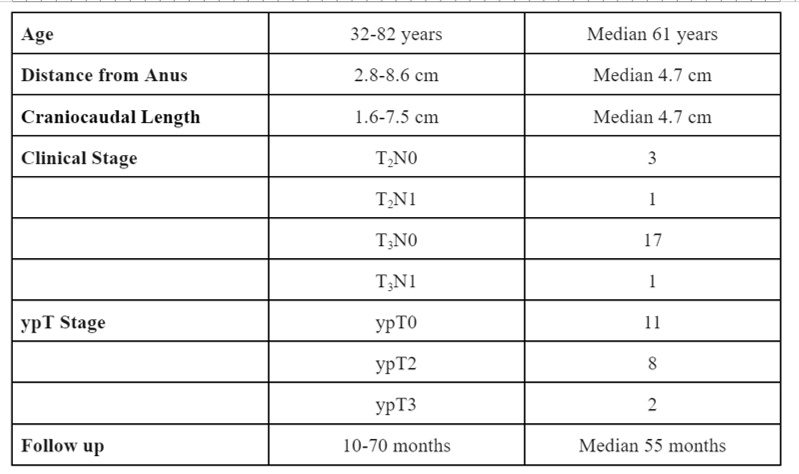 Table 1: Clinical parameters of patients who completed final restaging
Table 1: Clinical parameters of patients who completed final restaging
Back to 2024 Abstracts
