Back to 2024 Abstracts
PATHOLOGICAL COMPLETE RESPONSE (PCR) TO DURVALUMAB PLUS 5-FLUOROURACIL, LEUCOVORIN, OXALIPLATIN AND DOCETAXEL (FLOT) IN RESECTABLE GASTRIC AND GASTROESOPHAGEAL JUNCTION CANCER (GC/GEJC): REGIONAL SUBGROUP ANALYSIS FROM THE PHASE 3 MATTERHORN STUDY
Molena, Daniela
1*, Janjigian, Yelena
2, Al-Batran, Salah-Eddin
3, Wainberg, Zev A.
4, Van Cutsem, Eric
5; Muro, Kei
6; Hyung, Woo J.
7; Wyrwicz, Lucjan
8; Oh, Do-Youn
9; Omori, Takeshi
10; Moehler, Markus
11; Garrido, Marcelo
12; Oliveira, Sulene C.
13; Liberman, Moishe
14; Castro Oliden, Victor
15; Bilici, Mehmet
16; Kurland, John F.
17; Xynos, Ioannis
18; Mann, Helen
18; Tabernero, Josep
191Division of Thoracic Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States; 2Gastrointestinal Oncology Service, Memorial Sloan Kettering Cancer Center, New York, NY, United States; 3Institute of Clinical Cancer Research, Krankenhaus Nordwest, University Cancer Center, Frankfurt, Germany; 4Department of Gastrointestinal Medical Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States; 5Department of Gastroenterology/Digestive Oncology, University Hospitals Leuven and KU Leuven, Leuven, Belgium; 6Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan; 7Department of Surgery, Yonsei University College of Medicine, Seoul, Korea (the Republic of); 8Department of Oncology and Radiotherapy, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland; 9Division of Medical Oncology, Department of Internal Medicine, Seoul National University Hospital; Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea (the Republic of); 10Department of Gastroenterological Surgery, Osaka International Cancer Institute, Osaka, Japan; 11Research Center for Immunotherapy (FZI), Johannes Gutenberg-University Clinic, Mainz, Germany; 12SAGA Clinical Trial Centre and Universidad Mayor, Santiago, Chile; 13Clinical Oncology, The Clinical Research Center, Northern Riograndense League Against Cancer, Natal, Rio Grande do Norte, Brazil; 14Division of Thoracic Surgery, Department of Surgery, Centre Hospitalier de l'Université de Montréal, Centre de Recherche du CHUM, Montréal, QC, Canada; 15National Institute of Neoplastic Diseases (INEN), Lima, Peru; 16Department of Medical Oncology, Atatürk University Faculty of Medicine, Erzurum, Turkey; 17AstraZeneca, Gaithersburg, MD, United States; 18AstraZeneca, Cambridge, None Selected, United Kingdom; 19Medical Oncology Department, Vall d’Hebron Hospital Campus & Institute of Oncology (VHIO), IOB-Quiron, UVic-UCC, Barcelona, Spain
BackgroundFLOT was established as a perioperative therapy for GC/GEJC following the Phase 2/3 FLOT4 study conducted in Germany, with a pCR rate of 16% (Al-Batran et al, Lancet Oncol 2016). The global MATTERHORN study (NCT04592913) showed a statistically significant improvement in pCR with perioperative durvalumab (D) + FLOT vs placebo (P) + FLOT in GC/GEJC at first interim analysis (Janjigian et al, ESMO Congress 2023). Subgroup analyses by region and country were completed to assess pCR rates with FLOT and benefit of D + FLOT across the global study population.
MethodsParticipants (pts) with resectable GC/GEJC were randomized 1:1 to D 1500 mg or P every 4 weeks (Q4W) on Day 1 plus FLOT Q2W on Days 1 and 15 for 4 cycles (2 doses of D or P and 4 doses of FLOT pre- and post-operative), followed by D 1500 mg or P on Day 1 Q4W for 10 further cycles. Randomization was stratified by Asia vs non-Asia. pCR (Modified Ryan; central review) was assessed in prespecified (Asia) and post hoc regional subgroups, including 6 countries with the highest numbers of randomized pts.
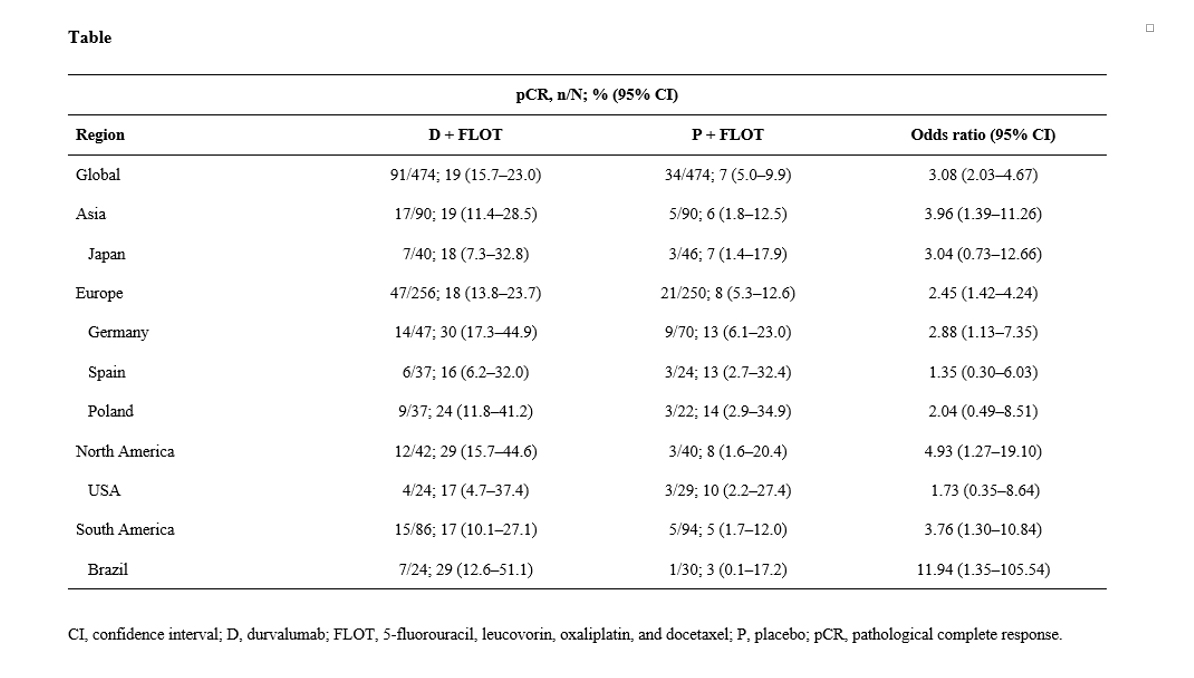
ResultsOf 948 pts randomized globally, 180 pts (19%) were in Asia. pCR outcomes with FLOT in Asia were consistent with the global outcomes. pCR rates were improved with D + FLOT vs P + FLOT in all regions (Asia, Europe, North America and South America; Table), despite some imbalances in baseline characteristics and numerical differences in pCR rates by geographic location. The pCR rate with P + FLOT in the German subgroup (13%; 95% CI, 6.1–23.0) was similar to that with FLOT in the FLOT4 study. Improvement in pCR with D + FLOT vs P + FLOT was observed across subgroups by country. Similar trends across regional subgroups were observed for combined complete and near-complete response rate.
ConclusionsIn MATTERHORN, pCR was consistently improved with the addition of D to perioperative FLOT in GC/GEJC across geographic regions. The study is ongoing for the primary objective of event-free survival.
Back to 2024 Abstracts
