Back to 2024 Abstracts
RISK FACTORS FOR PULMONARY EMBOLISM IN POSTOPERATIVE PATIENTS: DEVELOPMENT OF A RISK ASSESSMENT TOOL FOR CLINICAL DECISION MAKING AND USE OF COMPUTED TOMOGRAPHY PULMONARY ANGIOGRAPHY
Ilaria Caturegli
*1, Biqi Zhang
1, Baokun Gu
2, Bidisha Das
1, Mark M. Hammer
1, Jiping Wang
11Brigham and Women's Hospital, Boston, MA; 2Stanford University School of Medicine, Stanford, CA
Background: The most widely known risk assessment tools to discern patients with significant risk of having a pulmonary embolism (PE) that warrant CT pulmonary angiography (CTPA) for further evaluation were created by studying all-comers presenting for emergency care. Although they were adapted to inpatient setting, the validity of the score systems for postoperative patients has not been established. Our study aimed to evaluate the validity of the existing PE risk assessment tools for the postoperative setting and to create a PE risk scoring system specifically for postoperative patients.
Methods: This is a single-center, retrospective cohort study conducted according to STROBE guidelines of adult patients who received a CTPA after but within 30 days of surgery between June and August 2021. Patients who underwent pulmonary artery thrombectomy (n=6), aborted procedures (n=3), or had more than one operation (n=1) were excluded. The following clinicodemographic factors were collected: age, sex, vital signs, electrocardiogram findings, symptoms of venous thromboembolism (VTE), VTE history, American Society of Anesthesiologists (ASA) physical status, VTE prophylaxis, anesthesia type, surgical specialty, and major versus minor surgery. A multivariate logistic regression for acute PE was performed with 80% of the eligible cohort with tachycardia, VTE symptoms, smoking status, VTE history, ASA class, intubation status, and surgical specialty. Validation was performed with the remainder of the cohort.
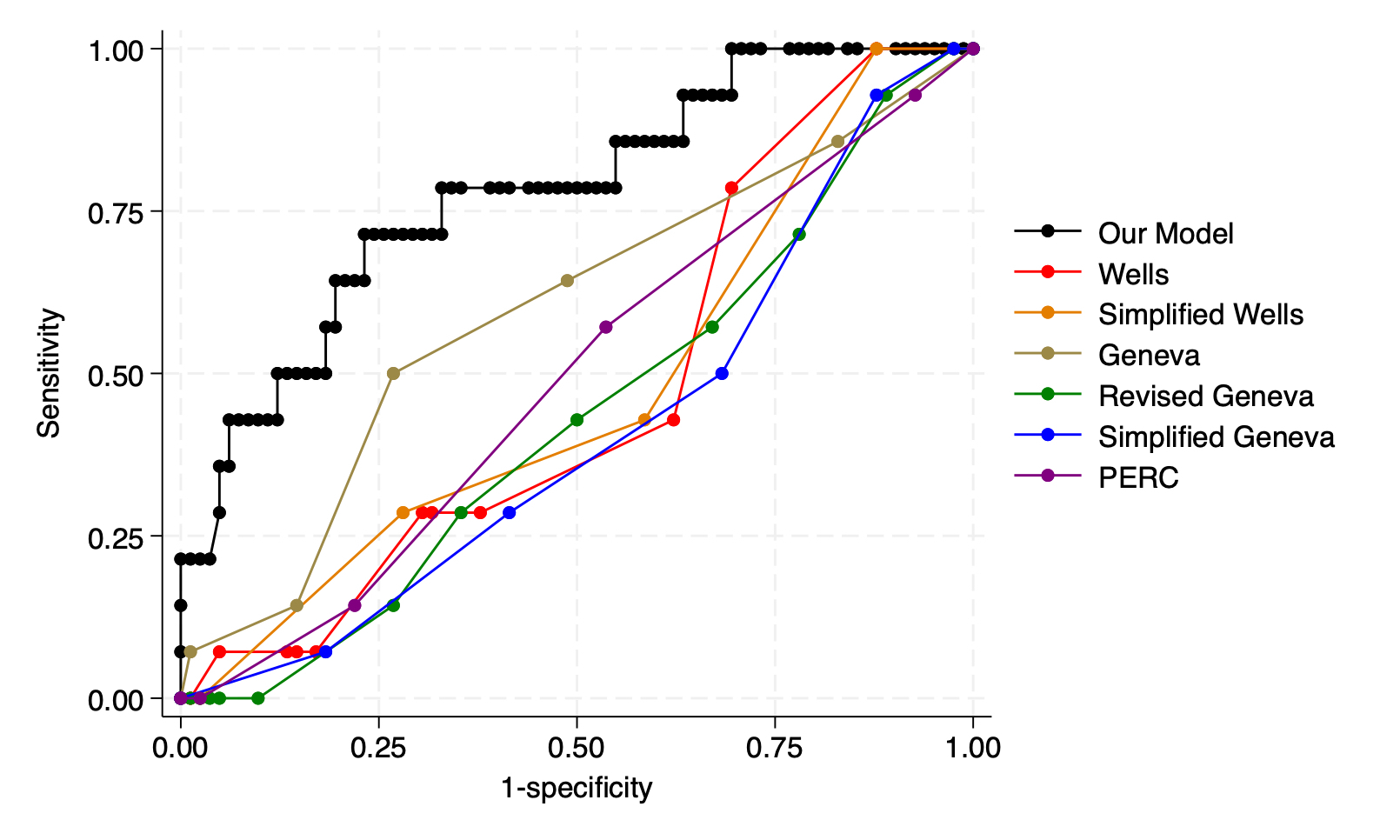
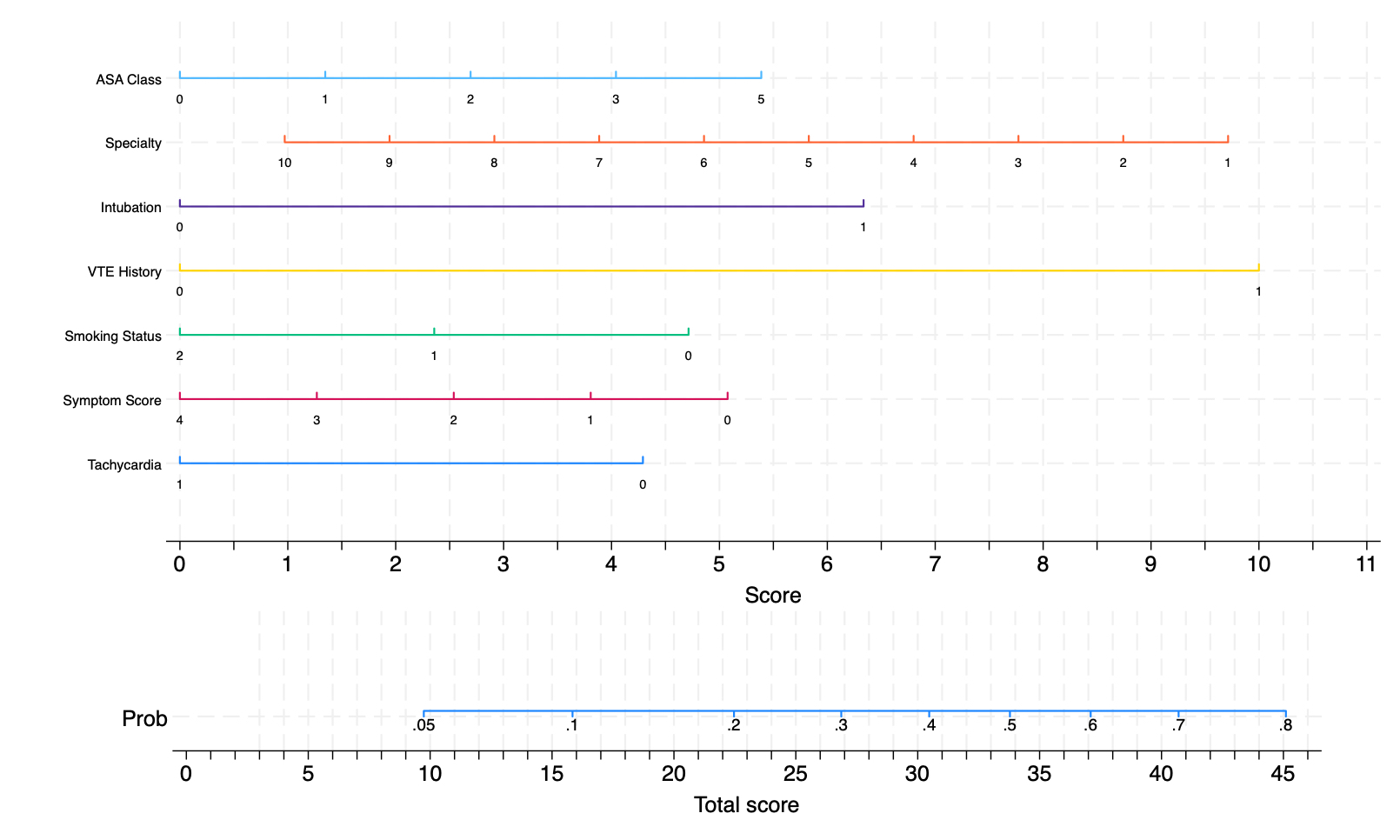
Results: 983 patients met study inclusion criteria, of which 168 (17%) had acute PE diagnosed on CTPA postoperatively. 49% of the cohort was male with median age 62 (IQR 50-72). 82% was white with median BMI 27.4 (IQR 23.5-32.4). 66% was within ASA class III and CTPA occurred at median 7 days (IQR 3-15) after surgery. Significant predictors of acute PE were history of VTE (OR=3.36, 1.97-5.73, p<0.05), intubation (OR=2.15, 1.13-4.12, p=0.020), and specialty (OR 0.89, 0.78-0.99, p=0.045) with area under the receiver operator curve (AUC) of 0.71 and 0.78 (0.64-0.91) for the training and validation data, respectively. In contrast, when applied to our population, Wells, Simplified Wells, Geneva, Revised Geneva, Simplified Geneva, and Pulmonary Embolism Rule-out Criteria had AUC of 0.47 (0.32-0.62), 0.48 (0.32-0.63), 0.59 (0.42-0.76), 0.44 (0.28-0.59), 0.41 (0.26-0.56), and 0.49 (0.34-0.64), respectively; and, p-value of <0.001 when compared to our model. A nomogram was created to predict risk of PE.
Conclusion: We propose an updated model based on clinicodemographic data to estimate the likelihood of PE in the postoperative population. This risk assessment model has significantly better accuracy for PE as compared to existing scoring tools in postoperative patients. External validation with an independent dataset is under investigation.
Back to 2024 Abstracts

