Back to 2024 Abstracts
CAUSES FOR REOPERATION AFTER GASTRIC NEUROSTIMULATOR IMPLANTATION
Katherine Englander
*, Samer Ganam, Tejasvi Paturu, Joseph Sujka, Vic Velanovich
USF, Tampa, FL
Introduction: The sole device to treat gastroparesis with neurostimulation approved for use is the Enterra
TM system (Enterra Medical, Inc., St. Louis Park, MN). The device is implanted in the subcutaneous tissue upon the abdominal fascia with electric leads implanted into the gastric musculature along the greater curvature. The Enterra
TM device has demonstrated effectiveness in over 50% of patients up to 10- years of follow up, however it has been observed that a secondary operation is frequently needed. The primary aim of our study was to describe common reasons for reoperation following Enterra placement. Additionally, we sought to describe the time interval between operations.
Methods: This is a retrospective chart review of 108 patients with symptomatic diabetic or idiopathic gastroparesis treated with Enterra device neurostimulator placement between 2012 and 2023. Records were reviewed for patient's operation dates and indication for repeated interventions. The indications for intervention were categorized into three groups: device-related complications, battery depletion, and ineffective symptom relief. The median number of reoperations, range of reoperation numbers, and time intervals between operations were calculated.
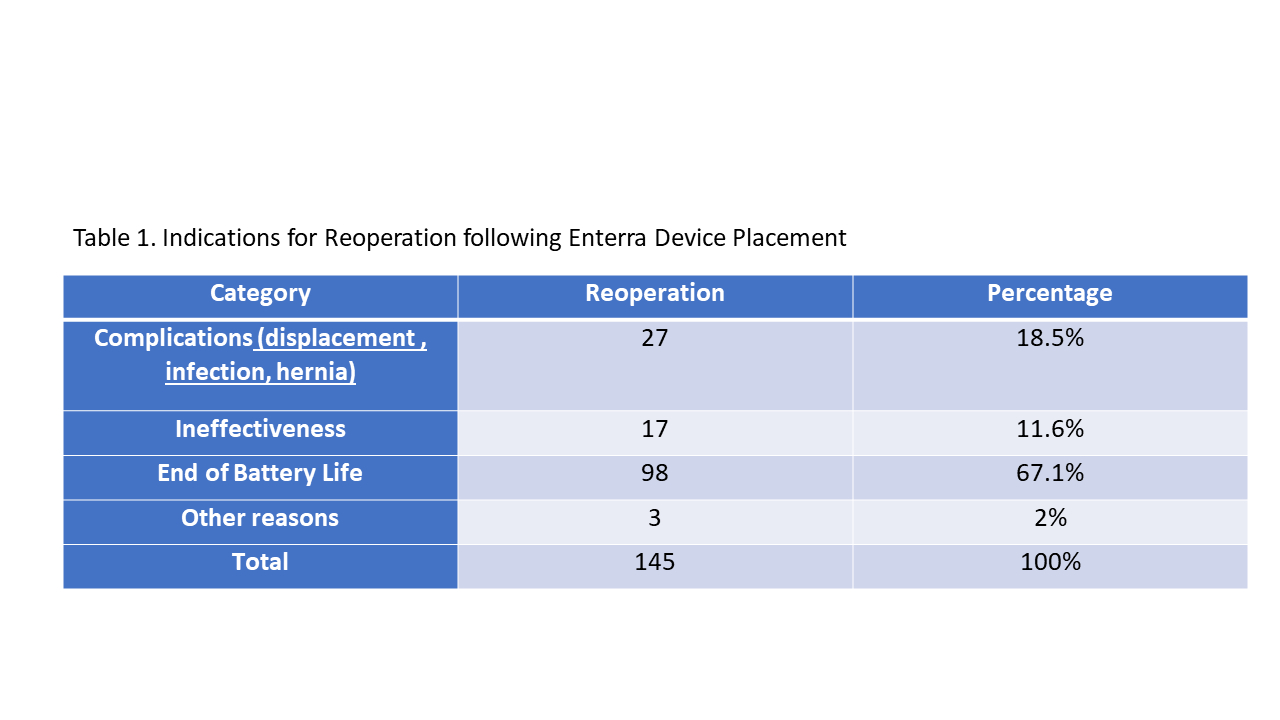
Results: Out of 108 patients, 57 (53%) required at least one reoperation. The median number of reoperations was found to be 2, ranging from 1-12 reoperations per patient. Among these reoperations, 27 (19%) were conducted due to complications such as displacement, infection, or hernia, while 17 (12%) were performed due to ineffectiveness resulting in persistent symptoms. The majority of reoperations, 98 (67%) were due to battery depletion, requiring device replacement. Other reasons for intervention include two cases who had the device removed for MRI and one patient who was checked for lead displacement which was found to be intact. The average time interval between operations was calculated to be 583 days (1.6 years) with a range of 12-2710 days (7.4 years)
Conclusion: This study revealed that a majority of patients who underwent Enterra device placement required reoperation. The primary reason for reoperation was the device's battery being depleted, causing recurrent symptoms. Although the average time in between operations was approximately 1.6 years, there was considerable variation. These findings can further inform presurgical counseling, emphasizing the need for on-going follow-up of this challenging group of patients. Further studies into those factors which result in reoperations including device settings, battery life, and placement techniques.
Back to 2024 Abstracts
