Back to 2024 Abstracts
CARCINOEMBRYONIC ANTIGEN INCREASE AFTER PREOPERATIVE CHEMOTHERAPY DISCONTINUATION PREDICTS RECURRENCE AND WORSE SURVIVAL AFTER RESECTION OF COLORECTAL LIVER METASTASES
Antony Haddad
*, Mateo Lendoire, Abhineet Uppal, Harufumi Maki, Reed I. Ayabe, Timothy E. Newhook, Yun Shin Chun, Ching-Wei D. Tzeng, Jean-Nicolas Vauthey, Hop S. Tran Cao
The University of Texas MD Anderson Cancer Center Division of Surgery, Houston, TX
Introduction: Carcinoembryonic antigen (CEA) levels may change after preoperative chemotherapy discontinuation before surgery for colorectal liver metastases (CLM). Whether preoperative CEA change is associated with recurrence-free (RFS), hepatic-specific disease-free (hDFS), and overall survival (OS) in patients with CLM is unclear.
Methods: Patients with initial curative-intent hepatectomy after preoperative chemotherapy for CLM (2004-2021) were identified. Patients without recorded CEA levels before, during, and after preoperative chemotherapy were excluded. Patients were stratified into three groups: normal CEA (<5.0 ng/mL) at all time points (CEA-normal); CEA increase (CEA-increase); and CEA decrease (CEA-decrease) after chemotherapy discontinuation.
Results: The study included 903 patients: 254 (28%) CEA-normal, 423 (47%) CEA-decrease, and 226 (25%) CEA-increase. CEA-decrease patients had similar starting pre-chemotherapy CEA (median 27.0 vs. 21.6 ng/mL) and minimum CEA (4.0 vs. 4.9 ng/mL), but lower final pre-operative CEA (median 4.0 vs. 10.1 ng/mL) vs. CEA-increase patients. On multivariable analysis, presence of extrahepatic disease (OR 1.98,
p<0.001) and a maximum CLM diameter >5 cm (OR 1.64,
p=0.013) were the only factors predictive of CEA-increase. Pre-chemotherapy CEA >55 ng/mL was not associated with CEA-increase.
Median RFS was 15.9 months for CEA-normal (reference), 12.2 months for CEA-decrease (
p=0.002), and 7.4 months for CEA-increase (
p<0.001) (Fig 1A). CEA-increase, but not CEA-decrease independently predicted RFS (HR 1.50,
p=0.002). Extrahepatic disease, multiple CLM, R1 resection, and
RAS-BRAF/
TP53 co-mutation also independently predicted worse RFS (Table 1). Median hDFS was not reached for CEA-normal but was 29.1 months for CEA-decrease (
p=0.003) and 14.8 months for CEA-increase (
p<0.001) (Fig 1B). CEA-increase, but not CEA-decrease independently predicted hDFS (HR 1.39,
p=0.03). Synchronous disease, multiple CLM, R1 resection, and
RAS-BRAF/
TP53 co-mutation also independently predicted worse hDFS (Table 1). Median OS was 11.9 years for CEA-normal, 7.1 years for CEA-decrease (
p=0.131), and 4.9 years for CEA-increase (
p<0.001) (Fig 1C). Only CEA-increase independently predicted OS (HR 1.79,
p=0.007). Right colon primary, extrahepatic metastases, multiple CLM,
RAS-BRAF/
TP53 co-mutation, and
SMAD4 mutation also independently predicted worse OS, whereas
APC mutation predicted better OS (Table 1).
Conclusion: CEA increase in the short interval between preoperative chemotherapy discontinuation and CLM resection is associated with worse oncologic outcomes, particularly in patients with
RAS-
BRAF/TP53 co-mutation, extrahepatic disease, and multiple tumors. This scenario should not necessarily prevent resection but may reframe patient and surgeon expectations in the final preoperative visit before CLM resection.
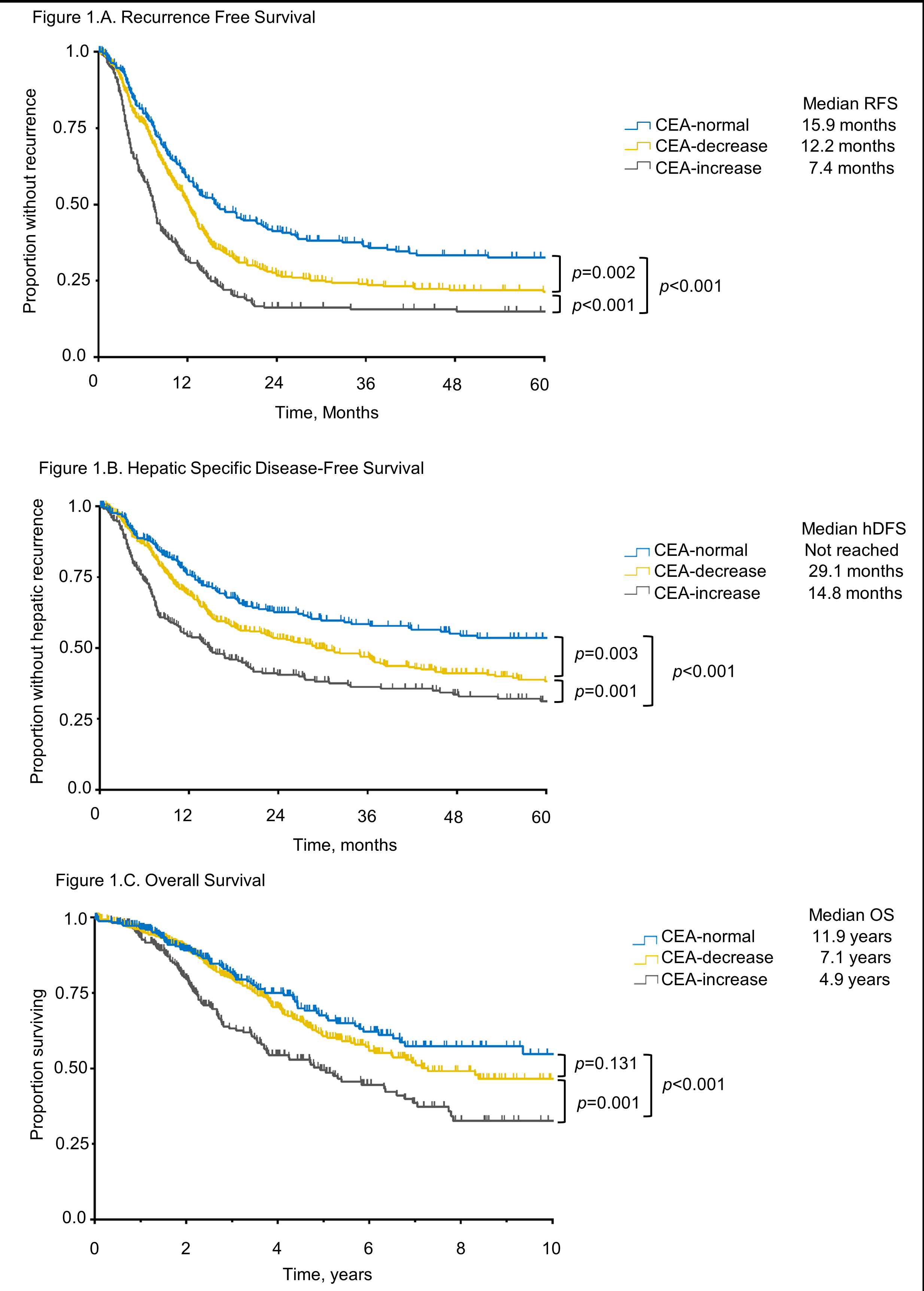
Figure 1.A. Recurrence-free, B. Hepatic specific disease-free, and C. Overall survival according to CEA variation
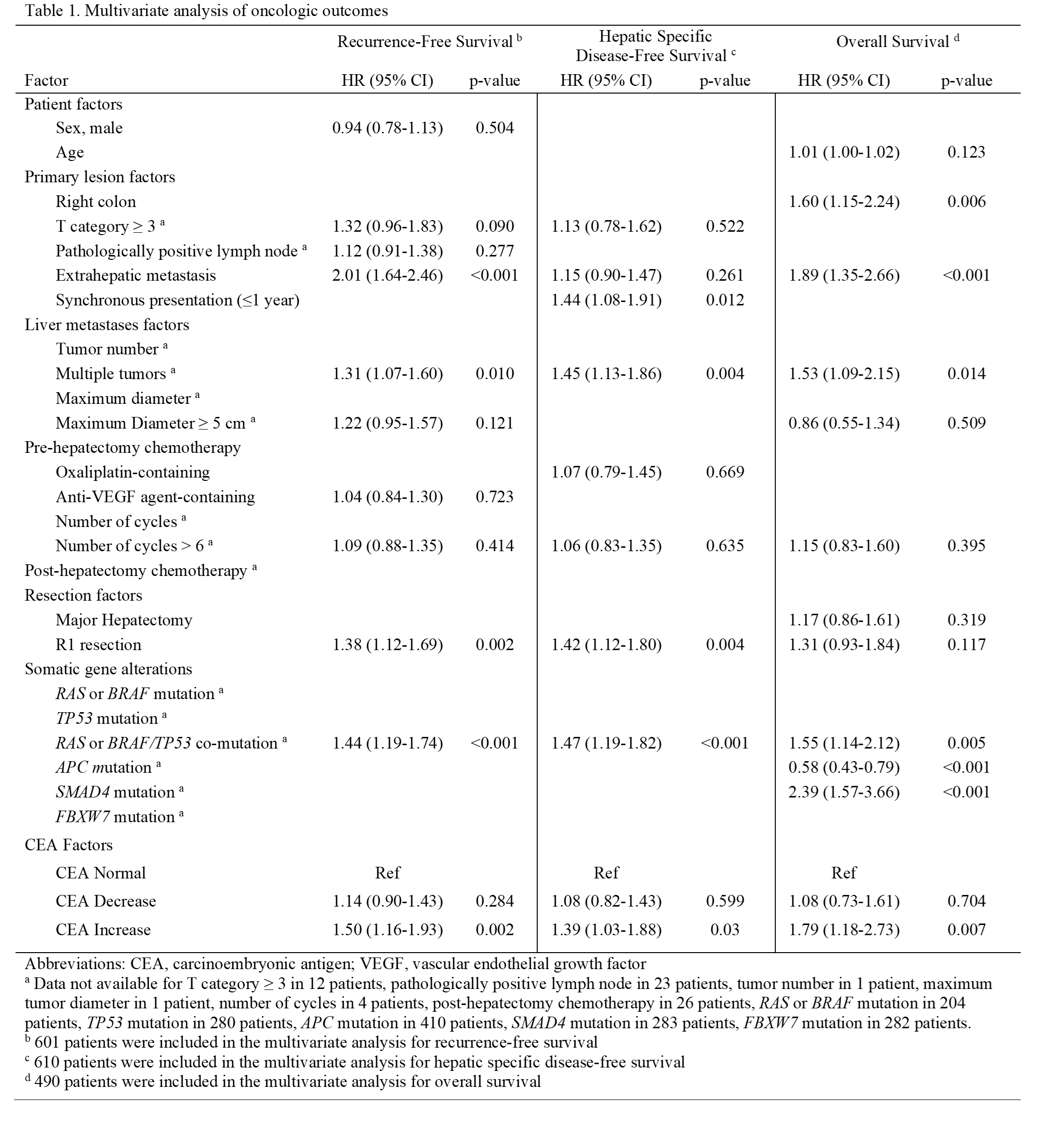
Table 1. Multivariate predictors of oncologic outcomes
Back to 2024 Abstracts

