Back to 2024 Abstracts
GRANULOCYTE-COLONY STIMULATING FACTOR(G-CSF) AND ERYTHROPOIETIN (EPO) COMBINATION THERAPY IN UPPER GASTROINTESTINAL BLEED WITH UNDERLYING LIVER CIRRHOSIS: A RANDOMIZED CONTROLLED TRIAL
Vishnu Prasad N R
*, Ankit Jain, Aishwarya R, Subrahmanyam DKS
Division of GI & HPB Surgery, Dept of Surgery, Jawaharlal Institute of Postgrduate Medical Education & Research (JIPMER), Puducherry, Puducherry U T, India, Puducherry, Pondicherry U T, India
Introduction: Management of variceal bleeding comprises a spectrum of measures. However, the risk of recurrence of variceal bleed persists till the primary cause of portal hypertension is dealt with. The definitive management for end stage liver disease and decompensated cirrhosis remains liver transplantation. However, owing to the regenerative ability of the liver, there has been a growing interest in novel regenerative therapies as a bridge to transplantation. Exogenous administration of cytokines to facilitate bone marrow stem cell mobilization for liver regeneration represents a simple and effective method. Therefore, this study was planned to study the effect of Granulocyte Colony stimulating factor (G-CSF) and Erythropoietin(EPO) in patients of upper GI bleed with underlying liver cirrhosis.
Objectives: Primary: Overall survival at 6 months in patients. Secondary objectives: 1) Number of rebleeding episodes 2) Development of new onset complications 3) Change in liver disease severity scores 4) Safety of the therapy
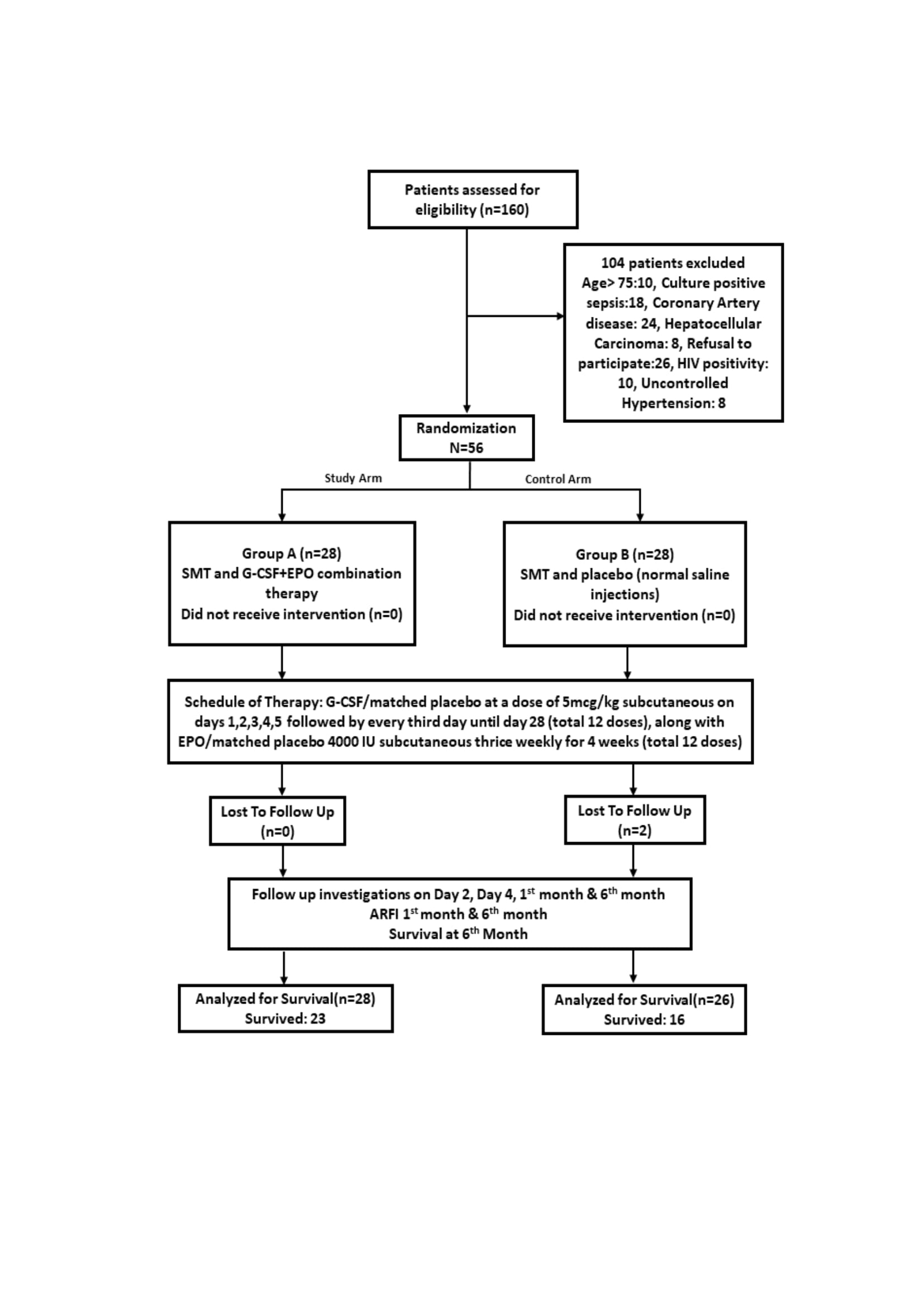
Methodology: This study was conducted in the Department of Surgery of a tertiary care hospital, India from January 2021 to February 2023. 56 patients above 18 years with UGIB and underlying liver cirrhosis were randomized to either receive Standard medical therapy (SMT) and G-CSF+EPO combination therapy [Group A] or placebo [Group B] in addition to Standard Medical therapy. Patients were followed up for 6months.
Results: The cumulative probability of survival at six months was 82.1% in group A and 61.5% in group B (P=0.091). Mean survival time was observed to be longer in Group A (158.5 ± 11.32 days) compared to Group B (129.6 ± 14.09 days) (P=0.124). mean %Δ change in MELD score, CTP score and MELD-Na score was higher in group B although not significant. There was significant decrease in the number of patients requiring hospitalizations, recurrent episodes of variceal bleed and SBP in Group A. No serious adverse effects warranting treatment discontinuation were noted following therapy with G-CSF+ EPO.
Conclusion: Use of combination therapy with G-CSF and EPO in patients with variceal UGIB, we observed longer survival times, though not amounting to statistical significance with a significant reduction in the number of hospitalizations and rebleed episodes. Safety of the combination therapy was also established with no serious adverse effects warranting treatment discontinuation.
Back to 2024 Abstracts
