Back to 2024 Abstracts
EPIGENETIC DYSREGULATION AND IMMUNE CELL INFILTRATION IN THE PANCREATIC NEUROENDOCRINE TUMOR (PNET) MICROENVIRONMENT: AN IMMUNOHISTOCHEMISTRY (IHC) ANALYSIS
Zena Saleh
*, Matthew C. Moccia, Hansa Joshi, Yazid K. Ghanem, Yahui Li, Upasana Joneja, Tao Gao, Young K. Hong
Surgery, Cooper University Health Care, Camden, NJ
Background: DNA Methyltransferase inhibitors (DNMTi) have been found to increase the immunogenicity of cancer cells, reshaping the immune tumor microenvironment while directly reprogramming immune cells. Combination therapy involving epigenetic and immune therapy has emerged as a highly effective treatment for multiple cancer types, including non-small cell lung cancer and melanoma. The characterization of the immune microenvironment in PNET and its response to immunotherapy is mainly unknown. We aim to investigate the interplay between epigenetic modifiers and immune infiltrates in PNET.
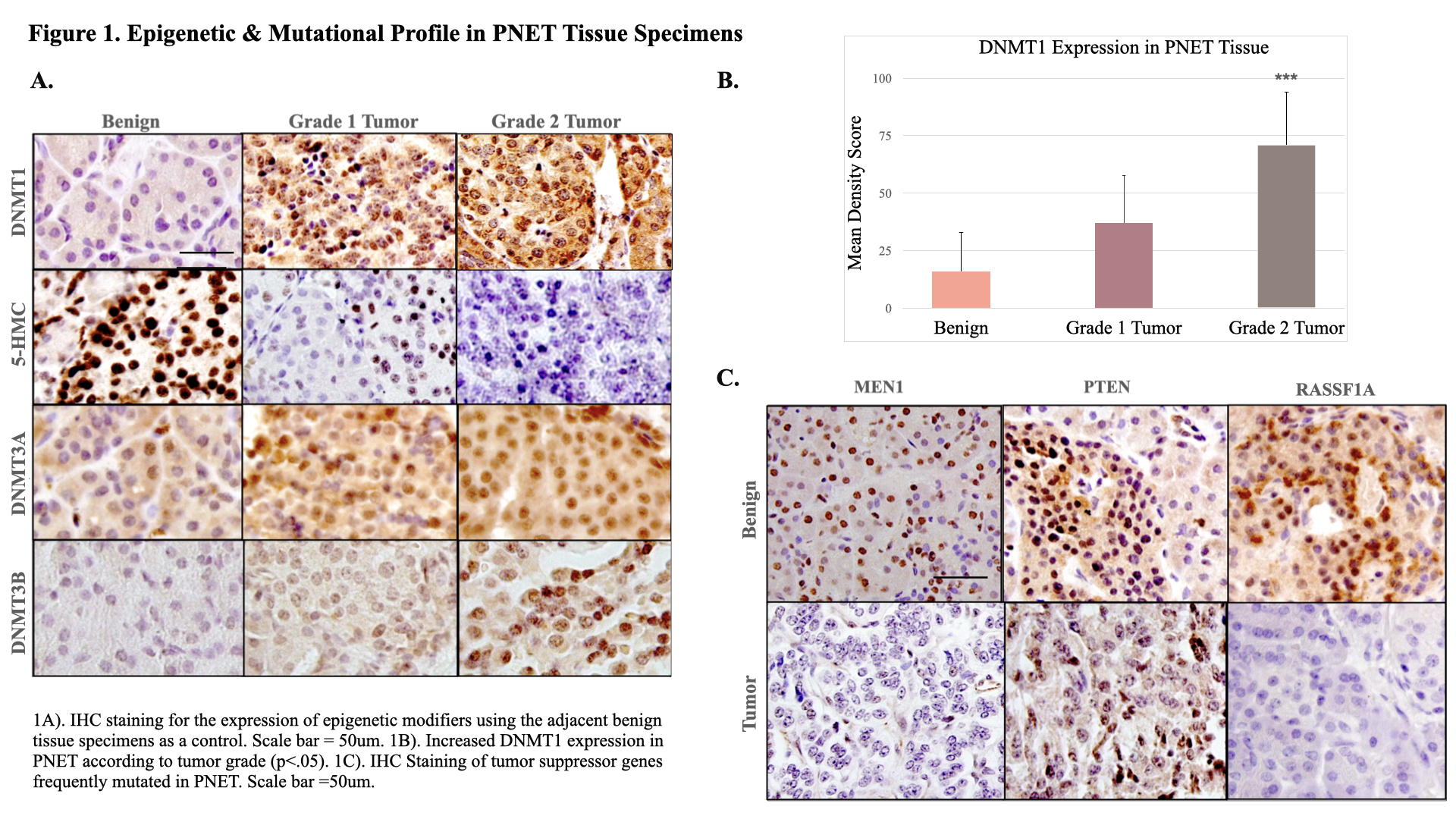
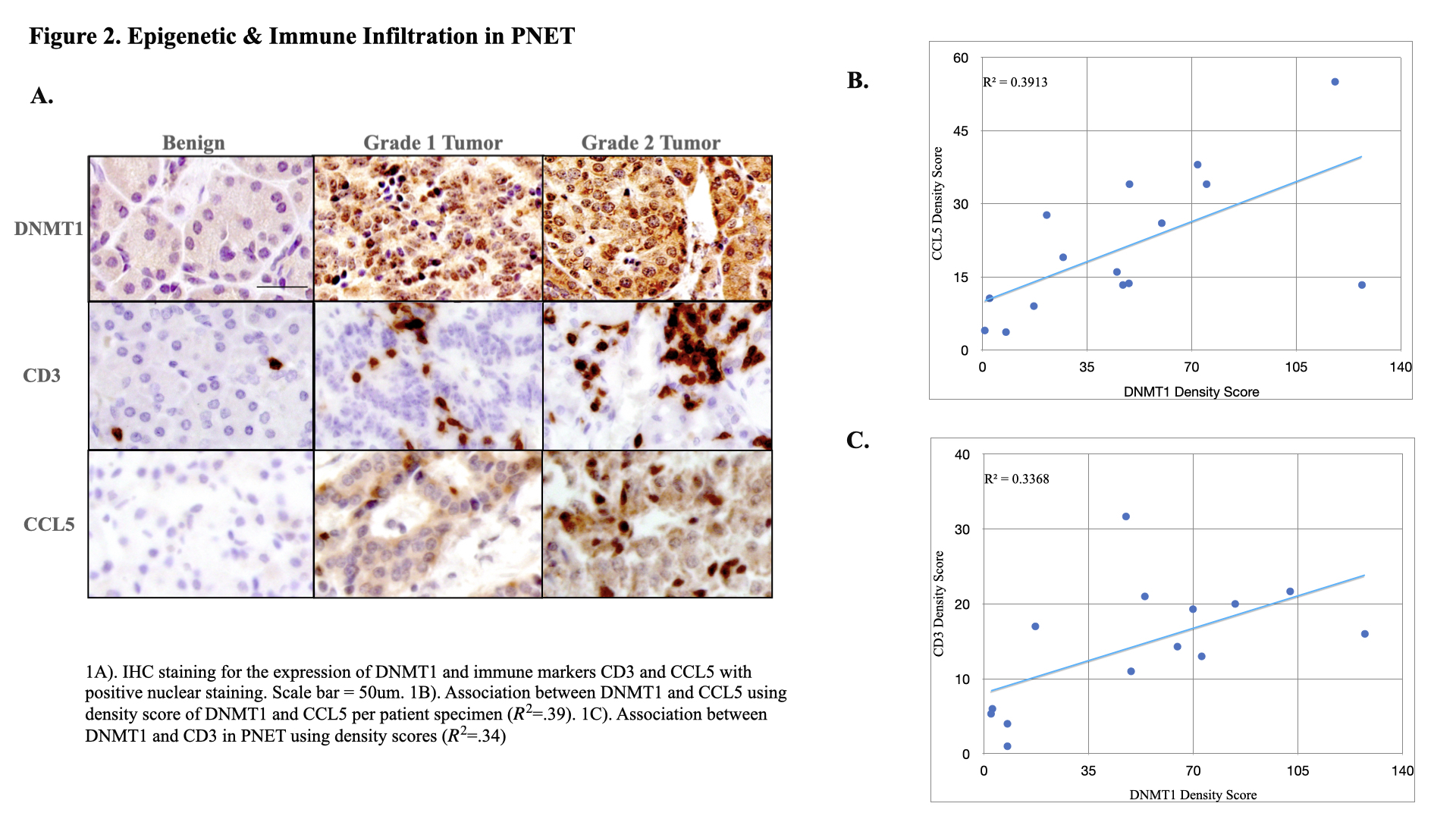
Method: 12 paraffin-embedded PNET specimens with their respective benign tissue specimens, used as controls, were obtained from Cooper University Hospital Pathology (IRB 23-130). IHC was performed using the antibodies DNMT1, DNMT3A, DNMT3B, 5-Hydroxymethylcytosine (5-HMC), Multiple Endocrine Neoplasia 1 (MEN1), Protein-Tyrosine Phosphatase (PTEN), Ras Association Domain Family 1 (RASSF1), Cluster of Differentiation 3 (CD3), and C-C Motif Ligand 5 (CCL5). IHC scoring was performed using Image J software under the review of a board-certified pathologist.
Results: Epigenetic dysregulation through DNMT1 was found to be upregulated in PNET (mean density score 72.02 +/- 31 in tumor versus 16.02 +/- 17 benign, ( p<.001)) and was directly associated with higher grade (mean 37 +/-21 in grade 1 vs. 71 +/- 23 in grade 2 (p<.05)). 5-HMC expression was decreased in tumor specimens (mean density 3.92 +/- 5.05 in tumor vs. 19.37 +/- 5.05 in benign, (p<.05)) and was found to be correlated with higher grade (mean .79 +/- 2 grade 2 vs. 8.2+/-2 grade 1, (p<.01)). DNMT3B expression was increased in PNET tumor specimen (mean 13.16 +/- 3.94 in tumor vs. 7.02+/- 2.553 in benign, (p<.01)) and was positively correlated to DNMT1 (R
2=.36). MEN1 was downregulated in PNET with 45% of tumor specimens displaying loss of MEN1 expression (mean density 4.47 +/- 5.95 vs. 11.41 +/- 3.53 in benign (p<.01)). Loss of RASSF1 expression was present in 70% of tumor specimens (mean 1.32 +/- 1.99 in tumor vs. 9.50+/- 6.89 in benign, (P<.001)). CD3 exhibited greater expression in tumor tissue in comparison to benign (mean 20.75 +/- 10.59 vs. 5.39 +/- 4.02, (p<.001)). CCL5 demonstrated increased uptake in tumor tissue (mean 20.36 +/- 12.4 vs. 5.3 +/- 4.8, (p<.001)) and was found to be directly associated with higher grade (mean 35.58 +/-10.9 in grade 2 vs. 18.45 +/-11.17 in grade 1). CCL5 and CD3 infiltration were positively correlated to DNMT1 (R
2=.39, R
2=.34) but had no mutational association with any specific PNET-related genes.
Conclusion: Our work provides a framework for the epigenetic and immune environment involved in the pathobiology of PNET, supporting the presence of tumor-specific immune responses that could be utilized for immunotherapeutic strategies.
Back to 2024 Abstracts

