Back to 2024 Abstracts
DNA-HYPOMETHYLATING AGENT DECITABINE COMBINED WITH CAPTEM CHEMOTHERAPY INDUCES REACTIVATION OF TUMOR SUPPRESSOR GENES VIA DOWNREGULATION OF DNMT1 EXPRESSION IN STC-1 NEUROENDOCRINE TUMOR CELLS
Matthew C. Moccia
*1, Chorlada Paiboonrungruang
2, Huan Li
1, Hansa Joshi
1, Yazid K. Ghanem
1, Zena Saleh
1, Tao Gao
1, Young K. Hong
11Surgery, Cooper University Health Care, Camden, NJ; 2Coriell Institute for Medical Research, Camden, NJ
Introduction: Pancreatic neuroendocrine tumors (PNETs) are a slow-growing, heterogeneous group of neoplasms arising from endocrine tissues of the pancreas. PNETs exhibit a low overall mutational burden, but the genes most commonly mutated are involved in epigenetic regulation. Therefore, we investigated the effect of an epigenetic therapeutic drug, DNA-hypomethylating agent decitabine (DAC), combined with standard chemotherapy, CAPTEM, on STC-1 neuroendocrine tumor cell cytotoxicity as well as tumor remodeling and its impact on tumor suppressor gene expression.
Methods: STC-1 cells were first plated at 1x10
4 in a 96-well plate and allowed to grow for 72 hours. They were then treated for 144 hours with CAPTEM chemotherapy (0.05µM 5-fluorouracil and 2.5µm temozolomide), a serial dilution of DAC starting at 1.2µM, or a combination of both chemotherapy and DAC. Viability was determined using IncuCyte® SX5 to select the optimal DAC concentration. Cells were then plated at 1x10
5 in a 6-well plate and again allowed to grow for 72 hours before triplicate treatment with either CAPTEM, DAC, or combination therapy. Cells were then collected from all wells, RNA and protein were prepared, and analysis for changes in known hypermethylated tumor suppressors was conducted via quantitative PCR and western blot.
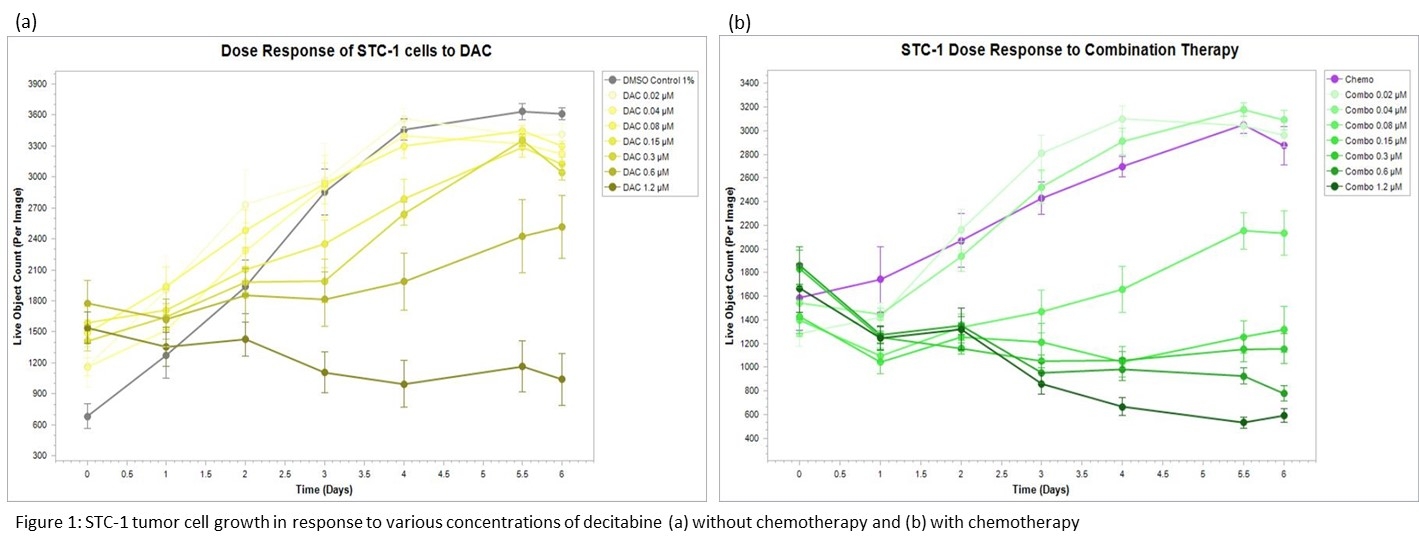
Results: DAC had a significant cytotoxic effect at higher doses, especially when combined with chemotherapy
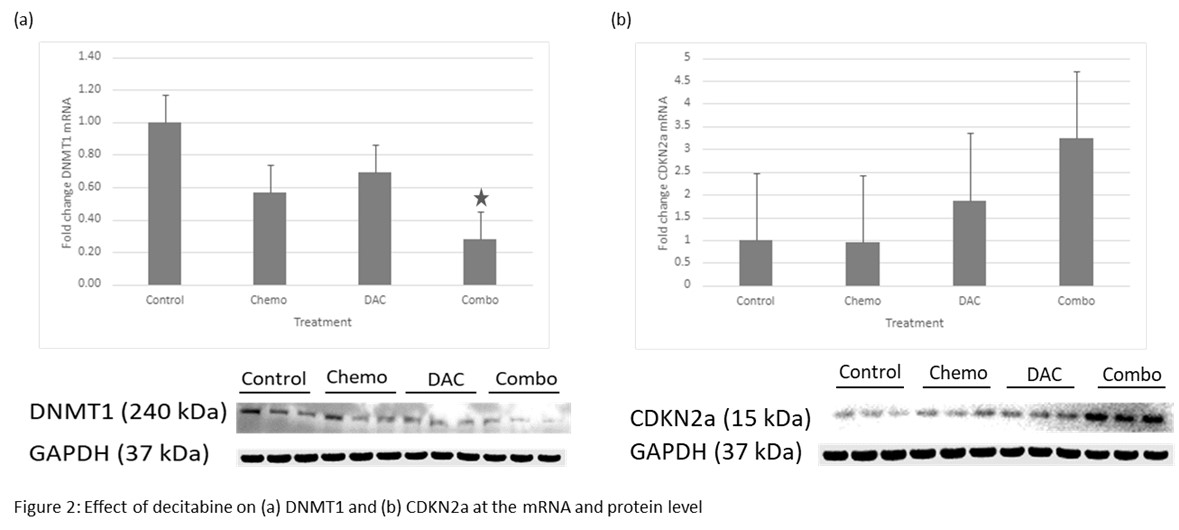
(Figure 1). Based on our dose-response curve, 0.075µM DAC was determined to offer the highest concentration without significantly increased cytotoxicity. After treatment, the combination group showed decreased DNMT1 at both the mRNA (72% reduction, p=0.047) and protein (62% reduction, p=0.028) levels. CDKN2a mRNA in the combo group was increased without statistical significance (224% increase, p=0.09), and protein level was increased in both the DAC (14% increase, p=0.032) and combo (38% increase, p=0.008) groups
(Figure 2). mRNA of two other tumor suppressor genes, RASSF1a and MGMT, was increased in both the DAC (92% increase, p=0.042 and 766% increase, p=0.049) and combo groups (245% increase, p=0.020 and 377% increase, p=0.018).
Conclusions: Decitabine induces reactivation of suppressed hypermethylated PNET tumor suppressor genes via downregulation of DNMT1 at both the RNA and protein levels. Our data suggests that, in some cases, DAC may work synergistically with standard-of-care CAPTEM chemotherapy. This indicates the potential for adding epigenetic therapies to current PNET treatment regimens.
Back to 2024 Abstracts

