Back to 2024 Abstracts
LARGE CALIBER ENDOSCOPIC BALLOON DILATION IN PATIENTS WITH PERSISTENT DYSPHAGIA AFTER MAGNETIC SPHINCTER AUGMENTATION (MSA) IS SAFE AND MAY REDUCE THE NEED FOR SUBSEQUENT DILATIONS AND DEVICE REMOVAL
Ifrah Fatima
*1, Mir A. Zulqarnain
1, Noor Hassan
1, Islam Mohamed
1, Adel Muhanna
1, Mohamed Ahmed
1, Abbas Bader
1, Sruthi Sripada
1, Kensey Gosch
2, B. Todd Moore
1,2, Sreenivasa S. Jonnalagadda
1,21University of Missouri-Kansas City, Kansas City, MO; 2Saint Luke's Health System, Kansas City, MO
Introduction: The magnetic sphincter augmentation device (MSA) is an emerging surgical therapy for refractory GERD which can be complicated by post operative dysphagia in up to 19% of patients. This can require endoscopic balloon dilation in up to 31% of patients with dysphagia and device removal in up to 5.1% of cases. The manufacturer's guidelines suggest dilatation after MSA should not be done within 6 weeks of implantation and should done with a through-the-scope balloon with a maximum diameter of 15 mm. We aimed to demonstrate the safety and efficacy of large-caliber (30mm) endoscopic balloon dilation in the management of persistent dysphagia after MSA placement.
Methods: This is a single-center retrospective review of all patients undergoing MSA placement from 2014-2023. Demographics (age, gender, BMI) and variables including size of MSA device, time to first dilation, repeat dilations, size of repeat dilations, complications (post dilation pain, need for hospitalization, perforation, need for surgery, need for device explant) were recorded. Patients who required esophageal balloon dilations were divided based on balloon size used- <20mm balloon size vs 30mm balloon size inflated to 8 PSI under fluoroscopic guidance. Chi-square and Fisher exact tests were used to compare categorical variables, and t-test for continuous variables. Multivariable logistic regression analysis was performed. A two-tailed
p value < 0.05 was considered statistically significant.
Results: 302 patients had MSA device placed of which 69 (22.8%) underwent subsequent esophageal dilation. 43 were dilated with a balloon size of < 20mm and 26 were dilated with 30mm balloon inflated to 8 PSI. Repeat dilation was more prevalent in those with an initial balloon size <20mm compared to 30 mm (41.9% vs 19.2%, p = 0.05). 2 patients had transient post dilation pain in the <20mm group and no other complications were seen in either group. There was a trend towards fewer dilations when the first dilation was done with 30mm balloon [OR=0.29 (95% CI 0.08-1.08), p=0.05). Device removal occurred in 16 patients (5.3%). Average time to removal was 14.5 months. Most common reason for removal was persistent dysphagia [11 (68.8%)]. Notably, only 3 (27.3%) of these patients had undergone dilation with the 30mm balloon prior to device removal.
Conclusions:
Endoscopic balloon dilation with 30 mm balloon inflated to 8 PSI appears to be safe in those with persistent dysphagia after MSA placement. There is a strong trend towards a decreased need for repeat dilations when the first dilation is done with a larger caliber balloon. Majority (72.7%) of the explanted devices had either been dilated with smaller balloon sizes or had not undergone dilation. Dilation with a large caliber dilation should be considered as first line in all patients experiencing dysphagia following MSA placement.
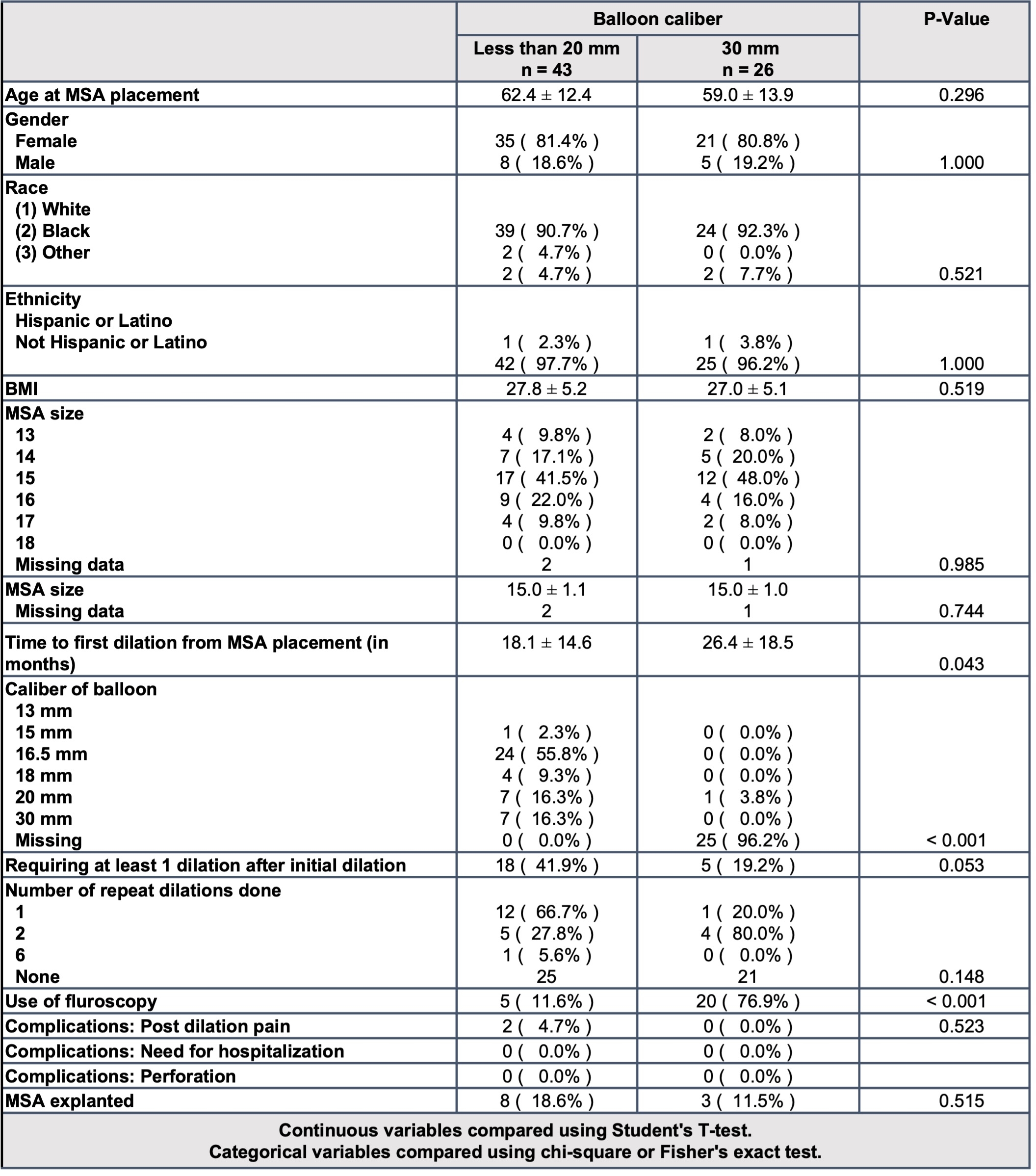 Table 1-
Table 1- Baseline characteristics of patients undergoing endoscopic balloon dilation with balloon caliber < 20mm vs 30mm
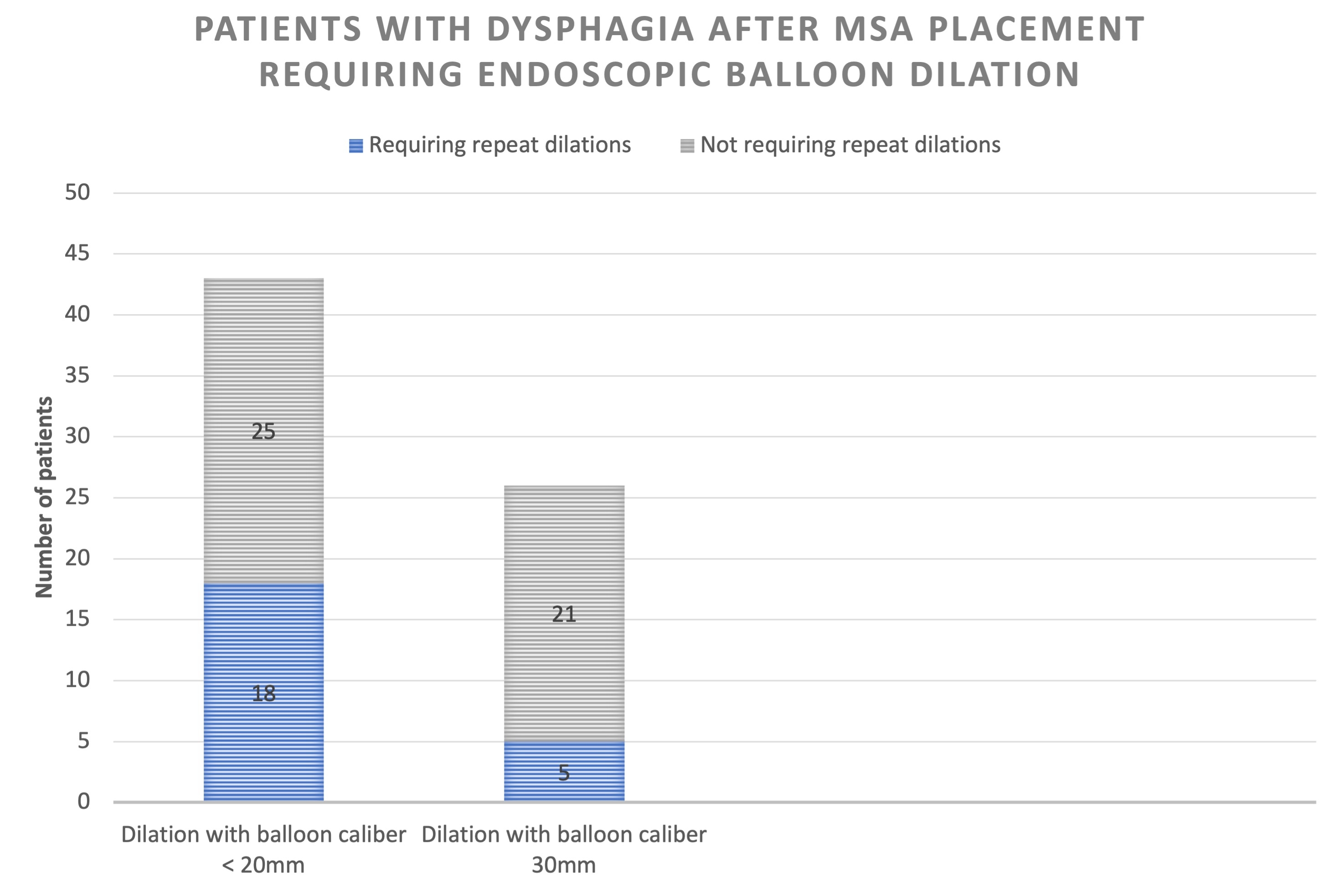
Figure 1- Patients with dysphagia after MSA placement requiring endoscopic balloon dilation
Back to 2024 Abstracts

