Back to 2024 Abstracts
A STRUCTURALLY ENGINEERED MEDIUM CHAIN FATTY ACID ANALOGUE AMELIORATES BILIARY FIBROSIS IN TWO MURINE MODELS OF PRIMARY SCLEROSING CHOLANGITIS
Scott C. Fligor
*1,2,3, Thomas Hirsch
1,3, Savas Tsikis
1,2,3, Amy Pan
1, Mikayla Quigley
1, Yury Popov
2,3, Mark Puder
1,31Boston Children's Hospital, Boston, MA; 2Beth Israel Deaconess Medical Center, Boston, MA; 3Harvard Medical School, Boston, MA
BACKGROUND: Few effective therapies exist to slow the progression of primary sclerosing cholangitis. Recently, we demonstrated that SEFA-6179, a structurally engineered medium chain fatty acid analogue, prevented cholestasis and reduced biliary fibrosis in a preterm piglet model of intestinal failure-associated liver disease. It is now in a human phase II trial. Here, we sought to extend our findings to other diseases of biliary fibrosis utilizing two murine models of primary sclerosing cholangitis with different mechanisms of injury. The
Mdr2-/- model is a knockout of the canalicular phospholipid transporter, resulting in phospholipid-free bile that results in chronic biliary injury. The 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) model leads to intraductal porphyrin plugs and cholestatic injury.
METHODS: Female wild-type and
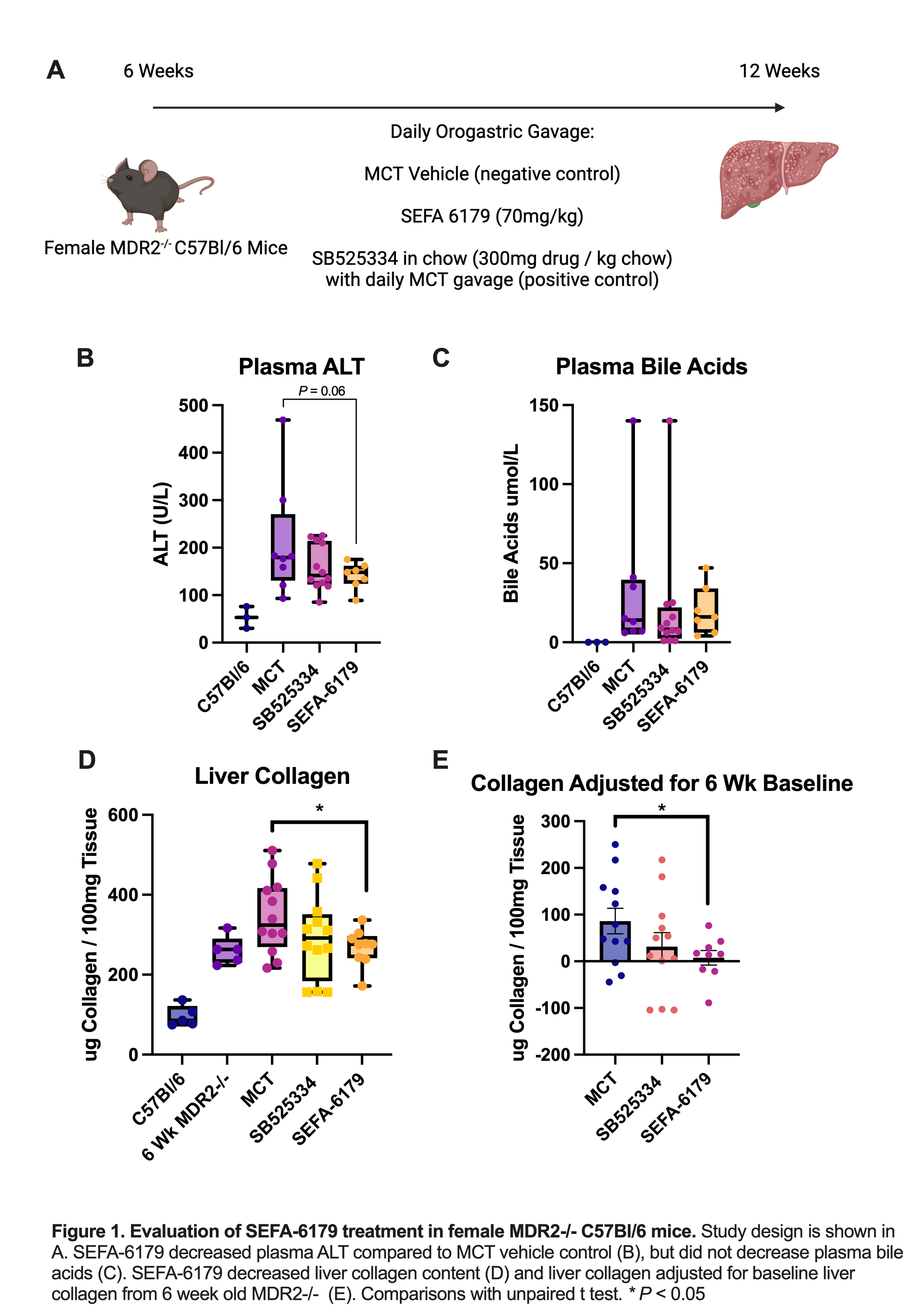
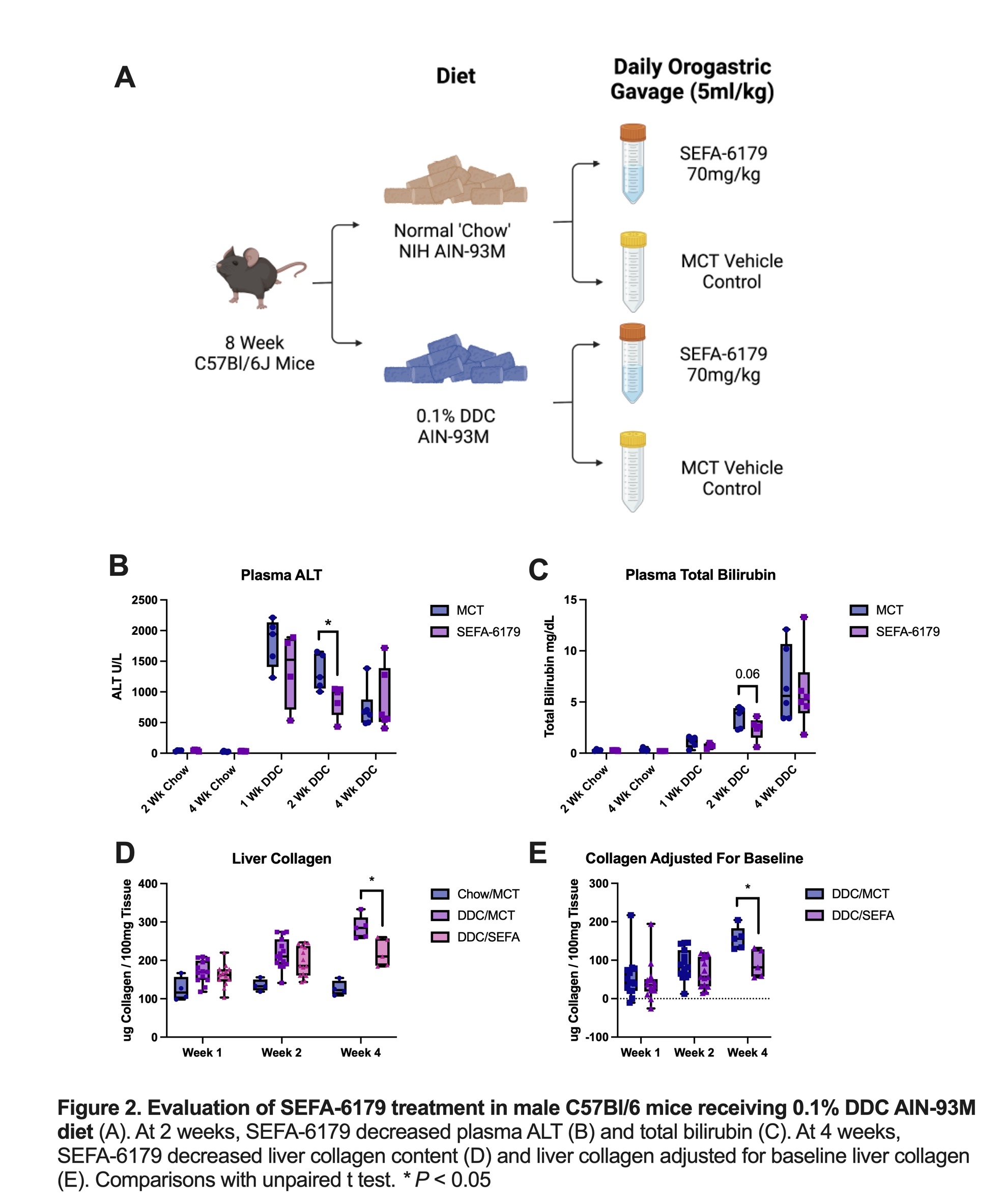
Mdr2-/- C57Bl/6 mice were treated with daily orogastric gavage from 6 to 12 weeks of life. Isovolumetric medium chain triglyceride (MCT) vehicle was a negative control. SEFA-6179 (70mg/kg/d) was the experimental treatment. A final group received SB525334 (TGF-B inhibitor) in chow (300mg drug/kg chow), a validated positive control in this model. Mice were sacrificed at 12 weeks. In the second model, 8-week-old male wild-type C57Bl/6 mice were administered either a ‘chow' diet (NIH AIN-93M) or chow supplemented with 0.1% DDC. Mice were treated with either MCT vehicle or SEFA-6179 (70mg/kg/d) via daily orogastric gavage. Mice were sacrificed at 1, 2, or 4 weeks. The primary outcome assessed was liver collagen content via a hydroxyproline assay.
RESULTS: In the first experiment (Fig. 1), SEFA-6179 decreased plasma ALT compared to MCT vehicle (141 vs. 211 U/L,
P=0.06) but did not affect plasma bile acids. SEFA-6179 decreased liver collagen content compared to MCT (268 vs. 347 ug collagen/100mg tissue,
P=0.04) and liver collagen adjusted for 6-week-old MDR2-/- baseline collagen content, which represents new collagen accumulation over the six week treatment period (8 vs. 79 ug collagen/100mg tissue,
P=0.02). In the second experiment (Fig. 2), SEFA-6179 treatment decreased ALT and total bilirubin at 2 weeks compared to MCT (ALT: 866 vs. 1320 U/L,
P=0.03; total bilirubin: 2.4 vs. 3.5 mg/dL,
P=0.15). At 4 weeks, SEFA-6179 treatment decreased liver collagen content compared to MCT vehicle (219 vs. 286 ug collagen/100mg tissue,
P=0.01), new collagen accumulation adjusted for baseline collagen in chow controls (91 vs. 157 ug collagen/100mg tissue,
P=0.01), and Sirius red staining (2.8 vs. 6.3% stained,
P=0.06).
CONCLUSIONS: In two murine models of primary sclerosing cholangitis with different mechanisms of liver injury, SEFA-6179 prevented the progression of liver fibrosis. Combined with our previous findings, SEFA-6179 ameliorates biliary fibrosis across multiple species and models, suggesting substantial translational potential.
Back to 2024 Abstracts

