Back to 2024 Abstracts
GOBLET CELL ADENOCARCINOMA AND RESPONSE TO TREATMENT - SINGLE CENTER EXPERIENCE OF A RARE MALIGNANCY
Jackson A. Baril
*1, Omer Saeed
1, Michael G. House
1, Eugene P. Ceppa
1, Ryan J. Ellis
1, Attila Nakeeb
2, Alexandra M. Roch
1, C. Max Schmidt
1, Nicholas J. Zyromski
1, Paul Helft
1, Trang Nguyen
11Surgery, Indiana University School of Medicine, Indianapolis, IN; 2Lahey Hospital and Medical Center, Burlington, MA
Introduction:
Goblet cell adenocarcinoma is a rare malignancy with an incidence of 1 per 2 million people worldwide. For patients with peritoneal spread, cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) may provide a survival benefit; however, there are limited data on treatment response given the rarity of the cancer. Therefore, this series aims to describe outcomes of patients treated for this rare malignancy.
Methods:
A retrospective cohort of patients with goblet cell adenocarcinoma was identified at a single tertiary referral center by searching a pathology database. Patients were stratified by stage at diagnosis and treatments received. Survival analyses were performed using Kaplan-Meier curves.
Results:
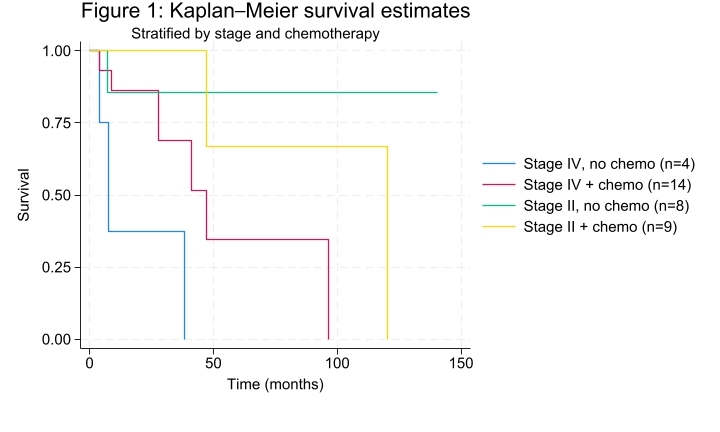
Forty-three patients with a histopathologic diagnosis of goblet cell adenocarcinoma undergoing treatment were identified between 2012 and 2023. Four patients had separate metastatic cancers at the time of diagnosis and were excluded, leaving 39 patients for the primary analysis. Of those, 49% (n=19) were female. At the time of diagnosis, 2.6% (n=1) had stage I, 44% (n=17) stage II, 8% (n=3) stage III, and 46% (n=18) stage IV disease. All 39 cases were primary tumors of the appendix and 23% (n=9) had signet ring features. After initial resection, 14 of 18 (78%) patients with stage IV, 1 of 3 (33%) patients with stage III, and 9 of 17 (53%) patients with stage II disease were treated with adjuvant chemotherapy, most commonly folinic acid, fluorouracil, and oxaliplatin (FOLFOX) (n=18, 75%). Eight patients with stage IV disease underwent cytoreductive surgery with a mean PCI score of 21.25 (range 4-33), and completeness of cytoreduction scores of CC-0 (n=3) and CC-1 (n=5). Seven (88%) of these eight patients had systemic chemotherapy before cytoreductive surgery and six (75%) also completed HIPEC. The median follow-up time was 26.4 months with median overall survival (OS) of 27 months. Five-year OS was 78% for stage II disease and 26% for stage IV disease (Figure 1). Among patients with stage IV disease treated with chemotherapy, 64% (n=9) had stable or radiographic response to chemotherapy. Of the six patients who underwent cytoreductive surgery with HIPEC, three are alive with disease and three have died at 9-, 41-, and 43-months post-diagnosis.
Conclusion:
Goblet cell adenocarcinoma remains a challenging disease. Patients with stage II disease have modest 5-year survival with surgical resection and selective use of adjuvant chemotherapy. Patients with peritoneal metastases can have long-term survival when treated with a combination of systemic chemotherapy, cytoreductive surgery, and HIPEC, though the role of the treatment itself versus careful patient selection remains unknown.
Back to 2024 Abstracts
