Back to 2024 Abstracts
PROMISING POTENTIAL FOR MITHRAMYCIN A, A NOVEL EPIGENETIC ANTINEOPLASTIC AGENT, IN CYTOREDUCTIVE SURGERY FOR PERITONEAL COLORECTAL CANCER
Yazid K. Ghanem
*, Matthew C. Moccia, Zena Saleh, Hansa Joshi, Weam Elbezanti, Young K. Hong
Surgery, Cooper University Health Care, Camden, NJ
Introduction:The benefit of hyperthermic intraperitoneal chemotherapy, in addition to cytoreductive surgery for colorectal cancer, is debated and warrants the assessment of novel therapeutic agents. Colorectal cancer is known to have aberrant epigenetic dysregulation to silence tumor suppressor gene expression. Herein, we aim to demonstrate the efficacy of FDA-approved epigenetic drugs compared to Mitomycin C as perfusional agents during cytoreductive surgery two-fold: their cytotoxicity and tumorigenic remodeling by reactivating tumor suppressor genes.
Methods: HCT116, human colon cancer cells, were treated with epigenetic drugs: Mithramycin A (MMA: antineoplastic antibiotic with epigenetic effects), Vorinostat (pan-HDAC inhibitor), Decitabine (DNMT inhibitor), and Tazemetostat (EZH2 inhibitor) and compared to Mitomycin C (MC). The doses tested for the drugs are between 0.1 µM and 20 µM. All the treatments in this study were performed for 90 minutes at 37 °C before removing the drug, and the media was changed before re-incubation. MTT Viability Assay was performed 48 hours post-treatment. The best-performing drug was then compared with Mitomycin C in the following areas: qPCR was performed to assess p21 and p53 gene expression 48 hours after treatment, a Wound Scratch Assay was performed to determine cell migration/invasion 72 hours after treatment, and cell colonies were stained 6-days after treatment to assess for their deconstruction and death.
Results:MMA IC50 0.685 µM was the lowest compared to the rest of the other agents MC (1.55 µM), Vorinostat (2.66 µM), Decitabine (7.34 µM), and Tazemetostat (13.46 µM) (P<0.05). MTT cell viability assay
(Figure 1) compares cell viability between the drugs tested 48 hours after treatment. qPCR revealed that treatment with MMA (0.7-1.5 µM) demonstrated a dose-dependent increase in p53 expression 1.22-1.69x, whereas MC (2 µM) downregulated p53 expression x0.53 (p-value <0.05). MMA also demonstrated a dose-dependent increase in p21 gene expression x1.73-2.41 (p-value <0.05), while MC (2 µM) increased p21 expression 2.96x (p-value <0.05). MMA also inhibited cell migration/invasion. Cells treated with MMA (3 µM) only migrated 36% (± 6.3%) over 72 hours, while cells treated with MC (10 µM) demonstrated 100% (± 0%) migration/invasion in the same period (p-value <0.05).
Figure 2 shows the effect of MMA compared to MC on colony growth.
Conclusions:We demonstrate a novel epigenetic agent, Mithramycin, with potent anti-proliferative and anti-invasion effects on colon cancer cells. MMA was also found to promote senescence by tumor remodeling with increased expression of p53 in treated cells. This warrants additional study to assess its potency for clinical application for local perfusion during cytoreductive surgery to improve not only cytotoxicity but also local recurrence by increased tumor suppressor genes.
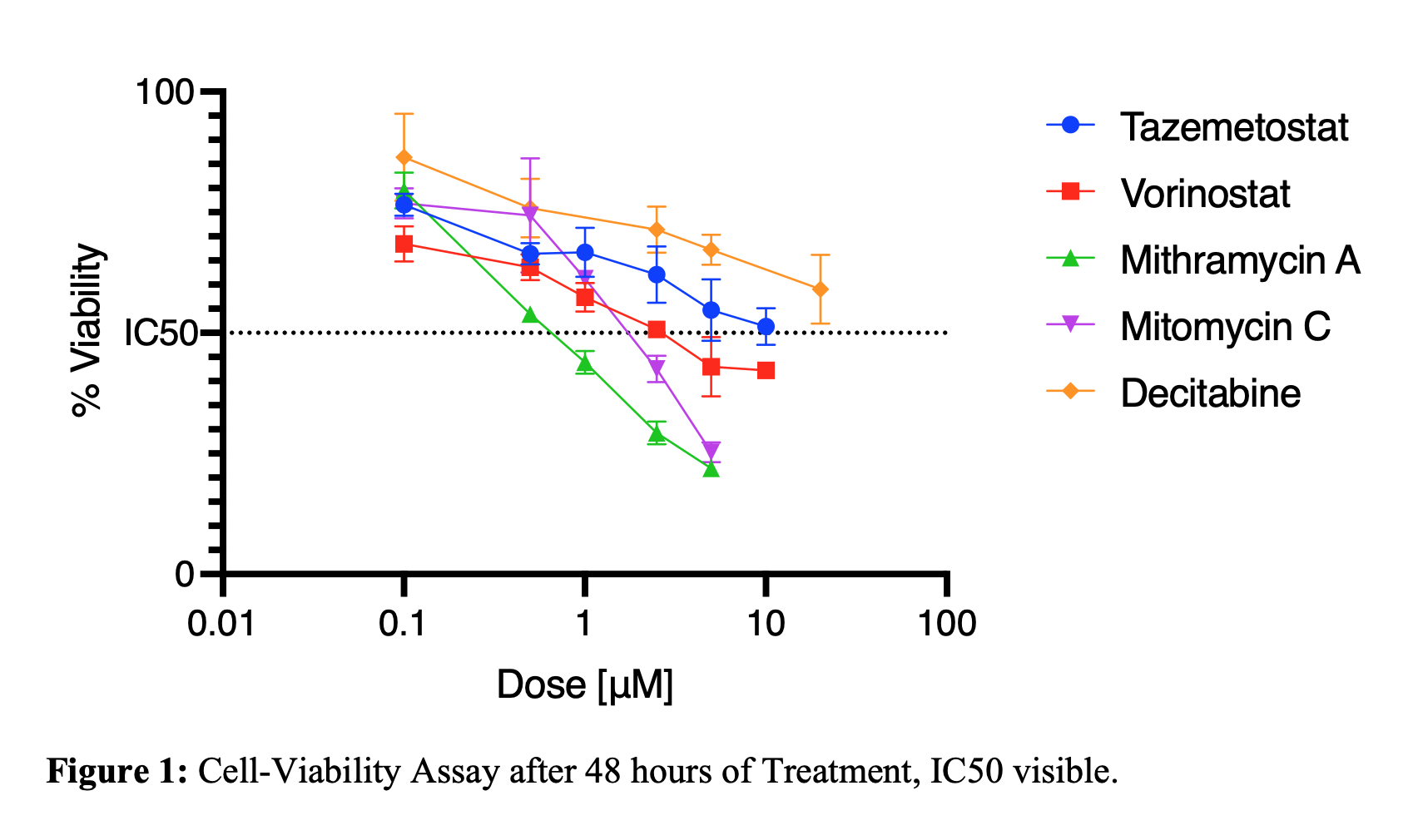 Figure 1:
Figure 1: Cell-Viability Assay after 48 hours of Treatment, IC50 visible.
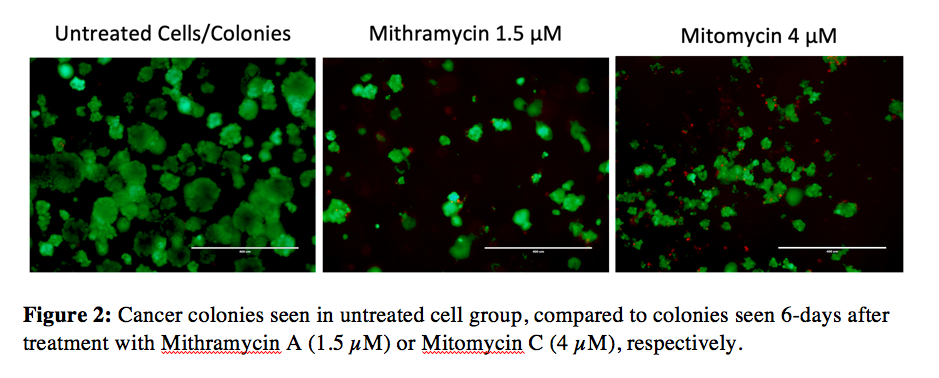 Figure 2:
Figure 2: Cancer colonies seen in untreated cell group, compared to colonies seen 6-days after treatment with Mithramycin A (1.5 µM) or Mitomycin C (4 µM), respectively.
Back to 2024 Abstracts

