PATIENTS WITH ADVANCED IPMC MAY BENEFIT FROM NEOADJUVANT CHEMOTHERAPY
Oliver Standring*, Neha Lad, Susana Benitez Sanchez, Lyudmyla Demyan, Gary Deutsch, Matthew Weiss, Danielle K. Deperalta
Surgical Oncology, Northwell Health, New Hyde Park, NY
Introduction
Intraductal Papillary Mucinous Carcinomas (IPMCs) are invasive neoplasms that arise from cystic tumors (IPMN). There is an increasing trend of utilizing neoadjuvant chemotherapy in conventional pancreatic adenocarcinoma, but this has not yet been described in IPMCs. However, as adjuvant chemotherapy becomes more common in IPMC treatment, we set out to explore the role of neoadjuvant therapy in IPMC.
Methods
A retrospective review of the National Cancer Database from 2004 to 2018 identified IPMCs using the histology code 8453. Only those with T1 disease or higher and who underwent surgical resection were selected for this study. Statistical significance was set at p< 0.05. The primary end-point was overall survival based on timing of chemotherapy using Cox regression analysis.
Results
960 surgically resected IPMCs were identified, of which 3.8% (37) received neoadjuvant chemotherapy (NAC), 39.7% (381) received adjuvant chemotherapy (AC), and 43.5% (542) underwent surgery exclusively (S). NAC and S groups were similar in demographics and Charlson-Deyo Comorbidity Condition Score (CDCC). The NAC group was more likely to have advanced AJCC stage II-IV disease (68.6% vs 28.7%, p<0.05) compared to the S group. There was no difference in the distribution of the lesion within the pancreas. Post resection, there were no significant differences in 30- or 90-day mortality, 30-day readmission rates, or negative margin rates between these two groups.
Controlling for age, CDCC, and T stage, there was a negative trend in overall survival for those who received neoadjuvant therapy (compared to S group) if node negative (Figure 1a, HR 1.393, CI: 0.722-2.688, p=0.322), but a positive trend in overall survival for those who received neoadjuvant therapy if node positive (Figure 1b, HR 0.515, CI: 0.242-1.098, p=0.086); individually, these did not reach statistical significance. However, in patients with T3- and T4-lymph node positive disease, there is a significant difference in survival in the NAC group (Figure 1c, HR 0.423 CI: 0.180-0.993, p= 0.048) compared to those who did not receive chemotherapy. As expected, adjuvant chemotherapy had a significant improvement in overall survival in all lymph node positive patients (Figure 1b HR 0.555, CI: 0.378-0.815, p=0.003) and in those with T3- and T4-lymph node positive disease (Figure 1c HR 0.507, CI 0.333-0.772, p=0.002).
Conclusion
Similar to recent advances in pancreatic ductal adenocarcinoma treatment, neoadjuvant chemotherapy may yet have a select role in treatment of IPMN, specifically in advanced, node positive tumors. This would require tissue diagnosis prior to neoadjuvant therapy, which is not currently common practice. Therefore, further studies are required to explore the tumor biology and clinical characteristics of those who truly may benefit from neoadjuvant chemotherapy prior to resection.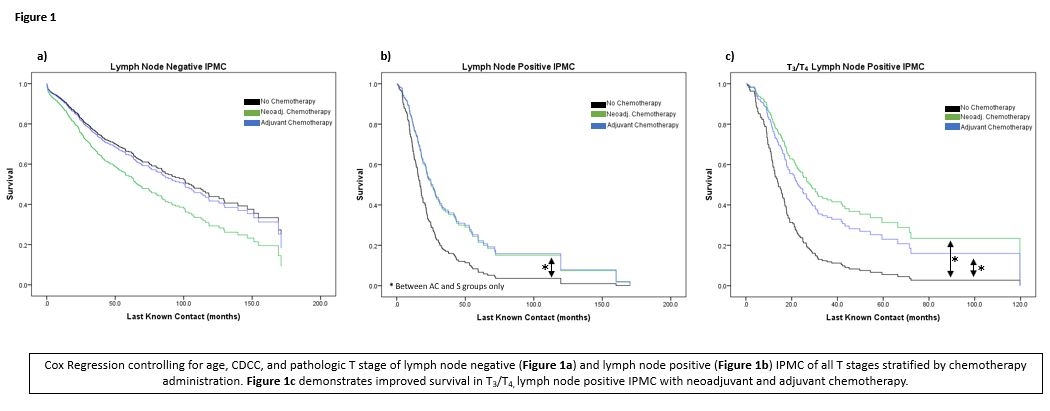
Back to 2022 Abstracts
