CYST FLUID GLUCOSE LEVELS ARE SUPERIOR TO CYST FLUID CEA LEVELS IN THE IDENTIFICATION OF MUCINOUS PANCREATIC CYSTS
Menghan Zhao*, Laura R. Prakash, Virginia Hill, Yi-Ju Chiang, Jeffrey Lee, William A. Ross, Emmanuel Coronel, Phillip S. Ge, Brian R. Weston, Jessica E. Maxwell, Timothy E. Newhook, Ching-Wei D. Tzeng, Naruhiko Ikoma, Jeffrey E. Lee, Matthew Katz, Manoop S. Bhutani, Michael P. Kim
Division of Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: The effective surgical management of pancreatic cysts hinges on the selective resection of mucinous cysts that are at high-risk for malignant progression. Cyst fluid carcinoembryonic antigen (CEA) levels are used to identify mucinous pancreatic cysts (MPC) from non-MPCs but suffer from low sensitivity, ranging from 25-75%. Cyst fluid glucose levels have been reported to better identify MPCs with sensitivities ranging from 88-95%. We sought to determine the concordance and combined accuracy of cyst fluid CEA and glucose levels at our institution to optimize the detection of MPCs for surgical management.
Methods: We identified all pancreatic cyst patients who underwent endoscopic fine needle aspiration (FNA) of pancreatic cyst fluid and subsequent quantification of CEA and glucose levels at our institution since 2019. MPCs and non-MPCs were designated using a combination of endoscopic data, cyst wall tissue analysis, and/or cross-sectional imaging. Categorical data using standard cut-off values for dichotimization were compared with Fisher's exact tests and numerical data were compared using Mann-Whitney U tests. Cyst glucose and CEA level association was analyzed using Spearman's rank correlation coefficient and Cohen's kappa coefficient.
Results: 57 patients were identified with cyst fluid CEA and glucose levels. 46 patients had MPCs (39 IPMN, 7 MCNs) and 11 patients had non-MPCs (pseudocysts, serous cystadenomas, mixed acinar neuroendocrine ductal carcinoma). Patient demographics and clinical data are listed in Table 1. The MPC group was predominantly female while the non-MPC group was predominantly male (p=0.01). Non-MPCs were larger compared to MPCs with a median size of 36mm vs. 22.5mm (p=0.02). The median cyst CEA level was higher in MPC patients compared to non-MPC patients (274 ng/mL vs. 1.4 ng/mL; p<0.001) while the median cyst glucose level was lower (29 mg/dL vs. 116 mg/dL; p<0.001). Only MPCs had a positive string sign on needle aspiration (40%) or mucinous epithelium present on cytopathology (50%).
The overall sensitivity and specificity of CEA ?192 ng/mL to identify MPCs were 56.8% and 100%, respectively. Cyst glucose levels ?50 mg/dL had an overall sensitivity and specificity of 94.9% and 90.9%, respectively, to identify MPCs. Cyst fluid CEA and cyst glucose levels demonstrated a fair correlation with a Spearman's correlation coefficient of -0.39 (p=0.006) and a Cohen's kappa coefficient of 0.27. Usage of either cyst CEA (?192 ng/mL) or cyst glucose (?50 mg/dL) levels generated an overall sensitivity and specificity of 100%.
Conclusion: Pancreatic cyst fluid glucose levels alone more accurately identify MPCs relative to cyst fluid CEA levels. However, cyst fluid CEA levels can be used in a complementary fashion to identify MPCs on rare occasions when cyst fluid glucose levels are inconsistent with other clinical data.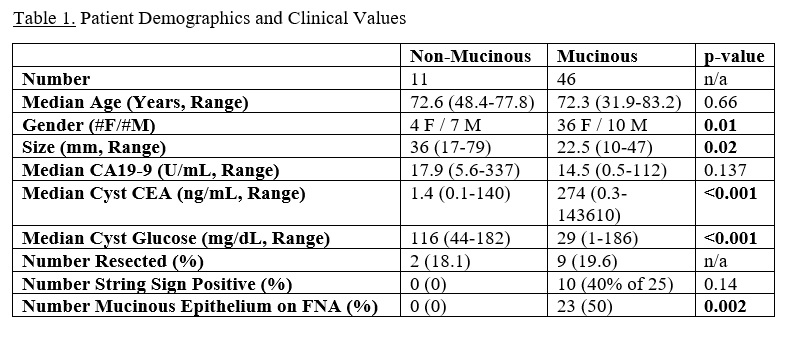

Back to 2022 Abstracts
