REAL-TIME MONITORING OF THE PATIENT EXPERIENCE DURING NEOADJUVANT THERAPY FOR GASTROINTESTINAL CANCER USING A CUSTOMIZED SMARTPHONE APPLICATION: PROOF OF FEASIBILITY
Christina Monsour, Angela Sarna, Lena Stevens, Emily Huang, Des D'Souza, Peter Kneurtz, Debasish Sundi, Heena P. Santry, Jordan Cloyd*
Surgery, OHIO STATE UNIVERSITY, Columbus, OH
Introduction: The delivery of non-surgical cancer therapies prior to surgical resection, known as neoadjuvant therapy (NT), is increasing for most solid organ cancers. Limited data is available on the patient experience or Health-Related Quality of Life (hrQOL) of patients undergoing NT. Given the limitations of traditional paper-based surveys administered at regularly scheduled physician appointments, we customized a smartphone app to prospectively measure the patient experience during NT.
Methods: A customized version of a commercially available mobile application was developed with engagement from patient and physician stakeholders. Participants were eligible if >18, English-speaking, owned a smartphone, and had not yet initiated NT. All recruitment, consenting, downloading/configuring the app, and patient counseling occurred remotely by research personnel. Previously validated surveys were "pushed" directly through the app: hrQOL was measured using Functional Assessment of Cancer Therapy (FACT) at baseline and monthly while surveys measuring treatment burden and care coordination were collected once. Participants were also encouraged to use the mood tracker, symptom tracker, and free-text journaling as often as possible. The study period continued until surgery was performed or cancer was determined inoperable.
Results: Between September 2020 and November 2021, of the 351 patients screened, 196 were eligible and contacted, and 99 (50.5%) participated in the study (Figure). The mean age was 60.58, and 56% were male. The most common cancer types were 40% colorectal, 35% hepatobiliary, 17% esophageal, and 2% gastric. Among all participants, hrQOL surveys were completed at baseline 73/93 (79%), one month 61/85 (72%), two months 36/80 (45%), and three months 22/76 (29%). 55 patients (81%) utilized the free-text journaling at least once and the average number of journal entries per participant was 24. Despite the observational nature of the study, positive therapeutic aspects of the program (journaling, education, feeling not alone, etc.) were frequently cited at completion of the study.
Conclusions: Preliminary results from this prospective observational cohort study suggest that real-time monitoring of the patient experience during NT can be obtained using a customized smartphone application although participation wanes over time. Modifications of the study protocol to be compliant with Covid-19 requirements enabled patient enrollment and data collection to be feasible entirely remotely. Analysis of the mature data should clarify opportunities to improve the patient experience and hrQOL during NT.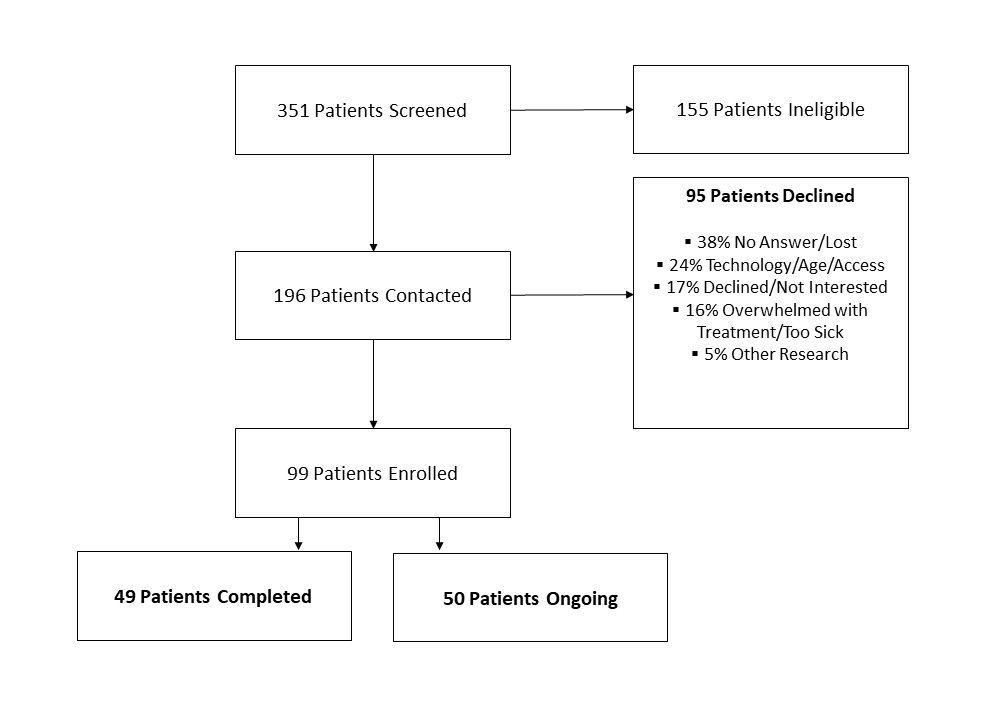
Back to 2022 Abstracts
