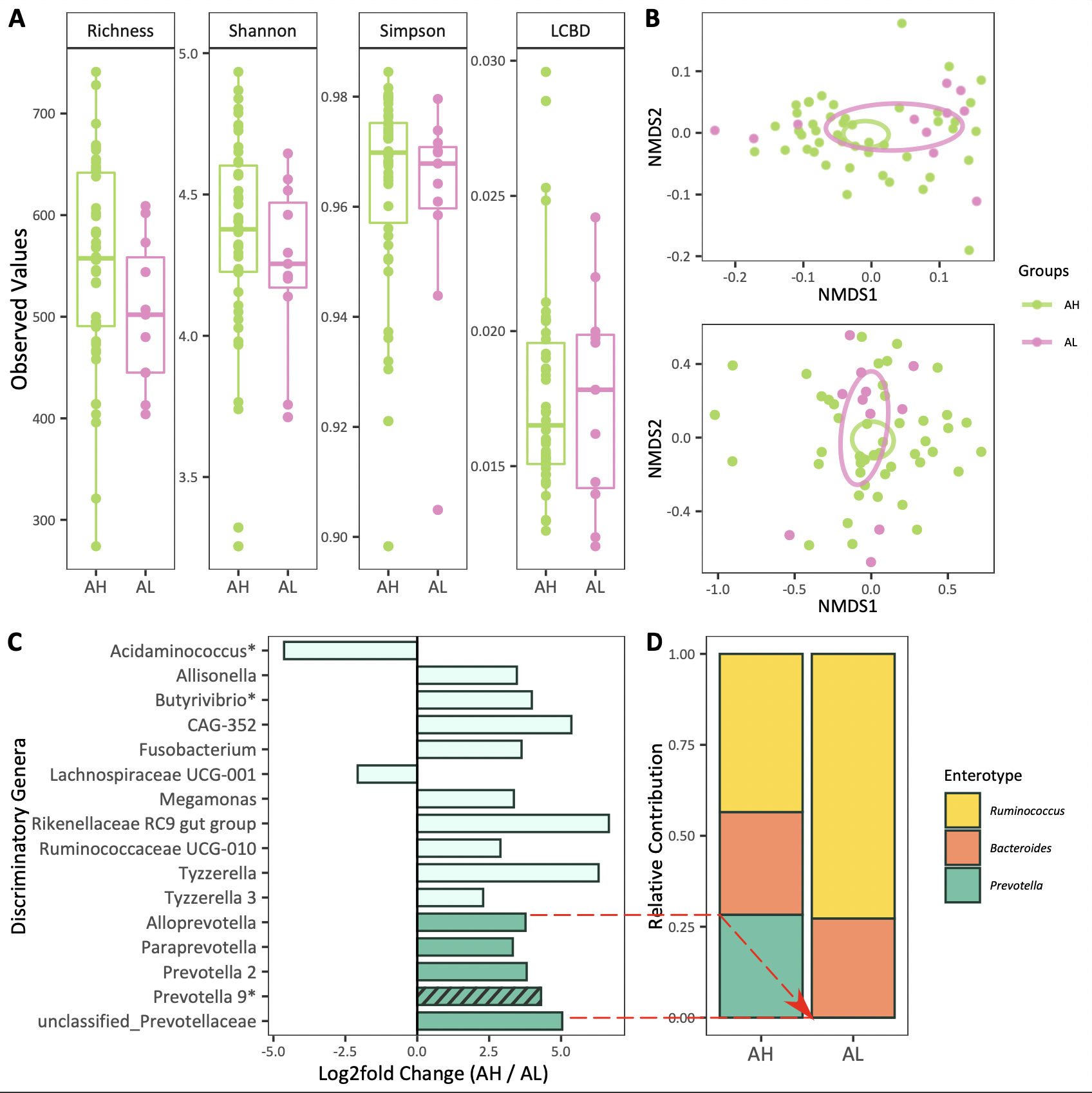
PREOPERATIVE ABUNDANCE OF PREVOTELLA ASSOCIATES WITH ANASTOMOTIC HEALING AFTER COLORECTAL SURGERY
Konstantina Zafeiropoulou*1,2, Emmeline G. Peters1,3, David J. Brinkman1,3, Andrew Y. Li Yim1,5, Boudewijn Smeets3, Theodorus B. Hakvoort1, Evgeni Levin4,6, Mark Davids4, Misha Luyer3, Wouter J. De Jonge1,7, Joep Derikx2
1Amsterdam UMC Location AMC Tytgat Institute for Liver and Intestinal Research, Amsterdam, Noord-Holland, Netherlands; 2Department of Pediatric Surgery, Emma Children's Hospital, Amsterdam University Medical Centers, Amsterdam, North Holland, Netherlands; 3Department of Surgery, Catharina Hospital, Eindhoven, Netherlands; 4Laboratory of Experimental Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands; 5Department of Clinical Genetics, Genome Diagnostics Laboratory, Amsterdam Reproduction and Development, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands; 6Horaizon BV, Delft, Netherlands; 7Department of General, Visceral, Thoracic and Vascular Surgery, University Hospital Bonn, Bonn, Germany
Background and Aims: Anastomotic leakage (AL) is a severe postoperative complication in patients undergoing colorectal surgery, which negatively impacts re-operation rates, duration of hospitalization, morbidity, mortality and quality of life. Although immunosuppressive medication and distal location of the anastomosis are known risk factors for AL, its pathophysiology remains poorly understood and options to prevent AL are limited. The current hypothesis suggests a multifactorial etiology, including host genetics and intestinal microbiota. In the present study, we aimed to unravel AL etiology and intestinal anastomotic healing (AH) physiology exploring both host transcriptional and gut microbial components.
Methods: We obtained 36 colonic tissue samples at time of surgery and 57 preoperative fecal samples from patients who underwent colorectal resection and got enrolled in the SANICS II trial. Colonic tissue-associated transcriptome was interrogated with the means of bulk RNA sequencing. Preoperative fecal samples were analyzed by 16S rRNA gene sequencing.
Results: Unsupervised exploratory analyses of the colonic tissue-associated transcriptome suggested no clear separation between AL (n=18) and AH (n=18 age-, sex- and body mass index-matched patients, Table 1) using hierarchical clustering analysis for the 150 most variable genes. Subsequent supervised analyses identified no difference in expression observed between AL and AH. Preoperative fecal microbiota ? diversity did not differ between AL (n=11) and AH (n=46, Table 1) groups (Figure 1A). Moreover, AL did not associate with the variation in fecal amplicon sequence variants community structure (P = .12, R2 = 2.8% and P = .58, R2 = 1.7% for weighted UniFrac and Bray-Curtis dissimilarity index, respectively, Figure 1B). At genus level, 16 taxa differed significantly between AL and AH, with 14 being significantly increased in AH (P (adjusted) < .05, Figure 1C). Importantly, five of the latter 14 (35.7%) taxa, including Prevotella 2, Prevotella 9, Alloprevotella, Paraprevotella and unclassified Prevotellaceae, are among the essential components of Prevotella enterotype (highlighted with dark green in Figure 1C), which in the present cohort was indicative of AH since no patients with a predicted Prevotella enterotype developed AL (P = .10, Figure 1D).
Conclusions: In the absence of host gene expression differences, the preoperative fecal microbiota of patients who undergo colorectal resection with successful AH differs in the group of patients who develop AL in the abundance of Prevotella and suggests a potential beneficial role of Prevotella that may prove useful as predictive biomarker for AH. A bilateral pipeline of research including larger-scale validation cohort and microbial manipulation animal work is currently pursued to confirm and further challenge the aforedescribed findings.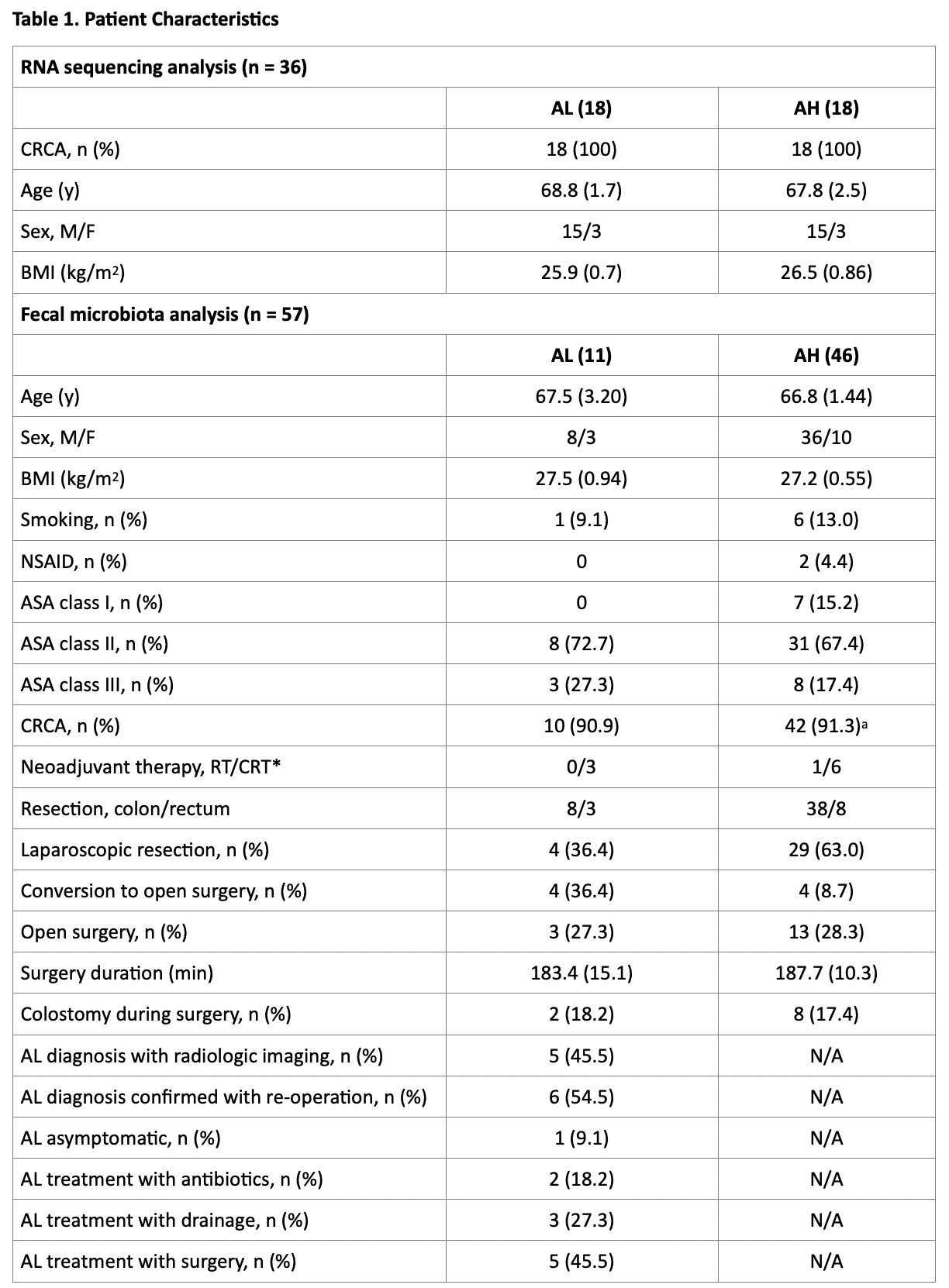
Back to 2022 Abstracts
