ANTI-REFLUX VERSUS CONVENTIONAL SELF-EXPANDING METAL STENTS (SEMS) IN THE PALLIATION OF ADVANCED ESOPHAGEAL CANCER: A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CLINICAL TRIALS
João Guilherme Ribeiro Jordão Sasso2, Igor M. Proença2, Epifanio S. Do Monte2, Igor Braga Ribeiro2, Alexandre M. Bestetti2, Angelo S. Kum2, Diogo T. De Moura2, Marcos Eduardo Lera Dos Santos2, Rodrigo S. Rocha2, Wanderley M. Bernardo2, Sergio A. Sánchez-Luna*1, Eduardo G. De Moura2
1Basil I. Hirschowitz Endoscopic Center of Excellence, Division of Gastroenterology & Hepatology, Department of Internal Medicine, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 2Universidade de São Paulo Hospital das Clínicas, Serviço de Endoscopia Gastrointestinal, Departamento de Gastroenterologia, São Paulo, Brazil
Background and Aims: Esophageal cancer is known as a pathology of fast progression and with a significant impact on the quality of life of patients. At the time of diagnosis, it is not uncommon for patients to not be eligible for curative treatment. Conventional self-expanding metal stents (SEMS-NV) are an effective palliative therapy to reduce dysphagia and improve the patient's nutritional status. Gastroesophageal reflux disease (GERD) is a common adverse event after the use of SEMS, and it has been proposed that valved SEMS (SEMS-V) could improve the quality of life of patients. This is a systematic review and meta-analysis that includes only randomized clinical trials (RCTs) and that aimed at comparing the efficacy and safety of anti-reflux versus conventional SEMS in the palliation of advanced esophageal cancer.
Material and Methods: A comprehensive search of multiple electronic databases to identify RCTs comparing SEMS-V and SEMS-NV, adverse events, SEMS migration, and technical success was performed following the PRISMA guidelines. The risk of bias was assessed by the Risk of Bias 2 (RoB 2) tool for randomized trials, data were analyzed with the Comprehensive Meta-Analysis V5.4 software, and quality of evidence by Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines.
Results: Ten RCTs evaluating a total of 467 patients, with 234 in the intervention group (SEMS-V) and 233 in the comparison group (SEMS-NV), were included in the statistical analysis. There were no statistically significant differences in post-procedure dysphagia (RD -0.07; 95% CI -0.19, 0.06; p = 0.30; I2=0%), post-procedural complications (RD 0.07; 95% CI -0.07, 0.20; p = 0.32; I2=59%), or technical success (RD -0.03; 95% CI -0.07, 0.01; p = 0.16; I2=0%) (Figure 1). Regarding stent migration, no significant difference was identified (RD 0.07; 95% CI -0.02, 0.15; p = 0.11; I2=0%) (Figure 2).
Conclusion: SEMS-V and SEMS-NV are both safe and effective therapies in the palliation of advanced esophageal cancer with no significant differences in post-procedural dysphagia, post-procedural complications, or technical success. Our study grouped SEMS-V with different anti-reflux valve mechanisms and therefore future randomized clinical trials are warranted to evaluate potential differences in GERD between different anti-reflux mechanisms.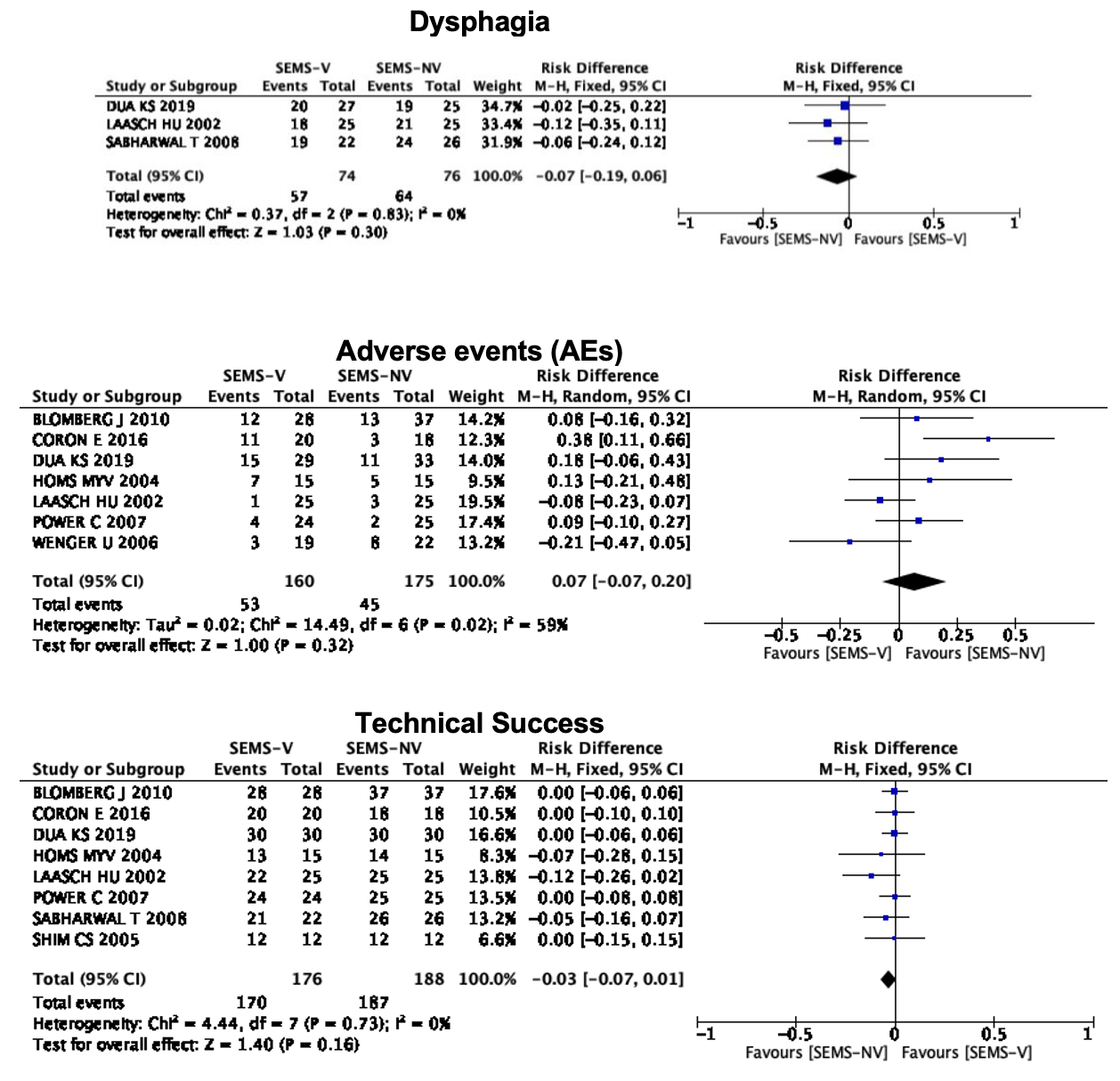
Figure 1. Plots for post-procedure dysphagia, post-procedural complications, and technical success.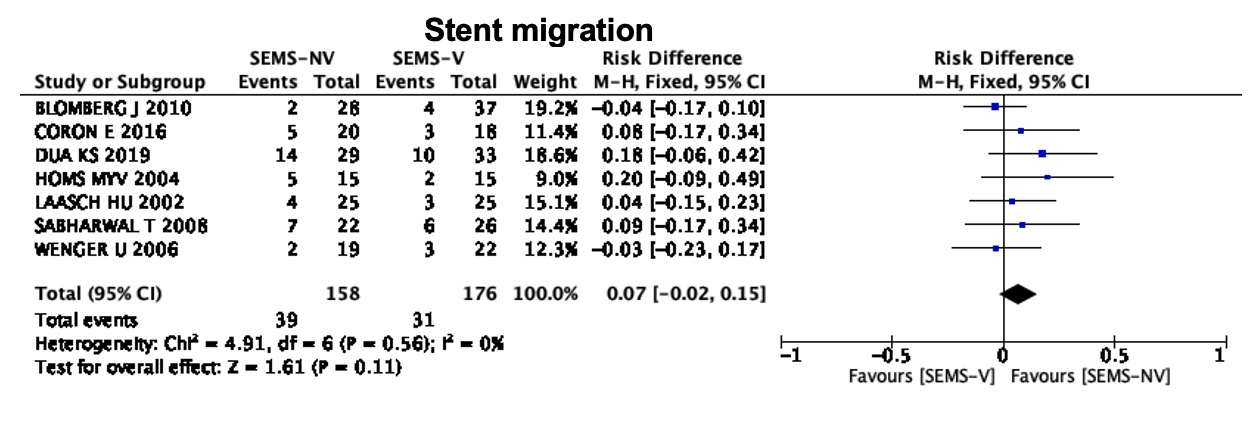
Figure 2. Plot for stent migration.
Back to 2022 Posters
