SEX-BASED CLINICOPATHOLOGIC AND SURVIVAL DIFFERENCES AMONG PATIENTS WITH PANCREATIC NEUROENDOCRINE TUMORS
Jacques A. Greenberg*, Nikolay A. Ivanov, Caitlin Egan, Yeon Joo Lee, Rasa Zarnegar, Thomas J. Fahey, Brendan M. Finnerty, Irene M. Min
Surgery, Weill Cornell Medicine, New York, NY
Introduction: Sex-based differences in survival trends have emerged among patients with pancreatic neuroendocrine tumors (PNETs). However, mechanisms driving these differences remain poorly understood. Among patients with PNETs, sex-specific transcriptional differences have been identified, as have mutational drivers with known prognostic significance. We aimed to further characterize sex-based clinicopathologic and survival differences and divergences in mutational signatures among patients with PNETs.
Methods: The National Cancer Database (NCDB) was queried for PNET patients diagnosed 2004-2017 who underwent surgical intervention. Clinicopathologic features were analyzed by sex. The overall survival of men and women by stage of disease was compared using Kaplan-Meier method. Sex-based differences in PNET mutational signatures were analyzed by querying the open access dataset of patients whose tumor genomic data was submitted to the American Association for Cancer Research Genomics Evidence Neoplasia Information (AACR-GENIE) Cohort v10.1-public. Frequencies of mutational signatures were compared by Fischer's exact (FE) test, adjusting for multiple testing via the Benjamini-Hochberg correction.
Results: 15,196 patients met inclusion criteria for analysis from the NCDB; 7,890 (51.9%) were men and 7,306 (48.1%) were women. Men more frequently had tumors > 2 cm than women (65.3% vs 60.1%, p<0.001) and more commonly had poorly or undifferentiated tumors (5.7% vs. 4.2%, p<0.001). Despite this, rates of lymph node positivity (29.1% vs. 28.1%, p=0.239) and distant metastases (11.3% vs. 10.9%, p=0.425) were similar. 5-year survival was worse for men than women (80.7, 95%-CI [79.7-81.7] % vs. 85.1 [84.1-86.0] %, p<0.001). However, this phenomenon appears to have arisen due to survival differences among patients with early stage disease and survival rates were similar among men and women who have stage 3 or 4 disease (stage 1: 88.2 [86.9-89.3] % vs. 92.1[91.0-93.1] %, p<0.001; stage 2: 77.1 [75.1-79.1] % vs. 81.0 [79.0-82.9] %, p=0.005; stage 3: 67.9 [55.2-77.7]% vs 69.6 [53.8-80.9]%, p=0.393; stage 4: 64.5 [60.6-68.2]% vs 66.3 [62.1-70.2]%, p=0.221).
DAXX, MEN1, PREX2, CDKN1B, GLI2, and HRAS mutations were more frequent among men with PNETs compared to women, whereas NRTK1, SPOP mutations were identified with more frequency among women (all p<0.05 by FE). However, none of these mutational differences maintained statistical significance when adjusted for multiple testing.
Conclusion: Compared to women, men have larger tumors but similar rates of distant metastases at time of diagnosis. Differences in overall survival between men and women appear driven by patients with early stage disease rather than those with more aggressive tumors, without clearly identifiable differences in mutational signatures between the sexes that may account for tumor aggressiveness.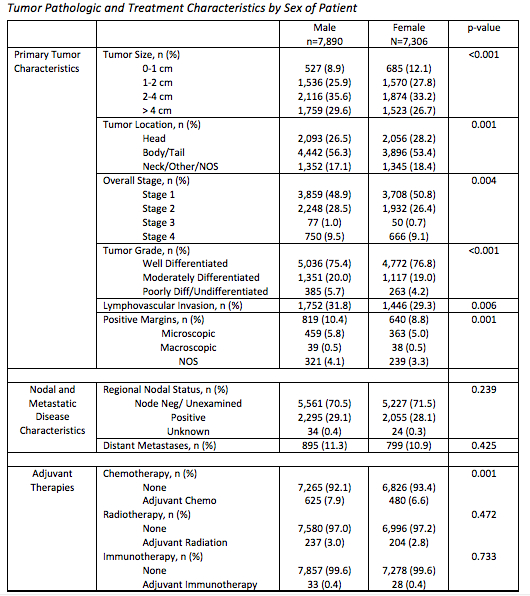
P-value compares men and women by chi-square test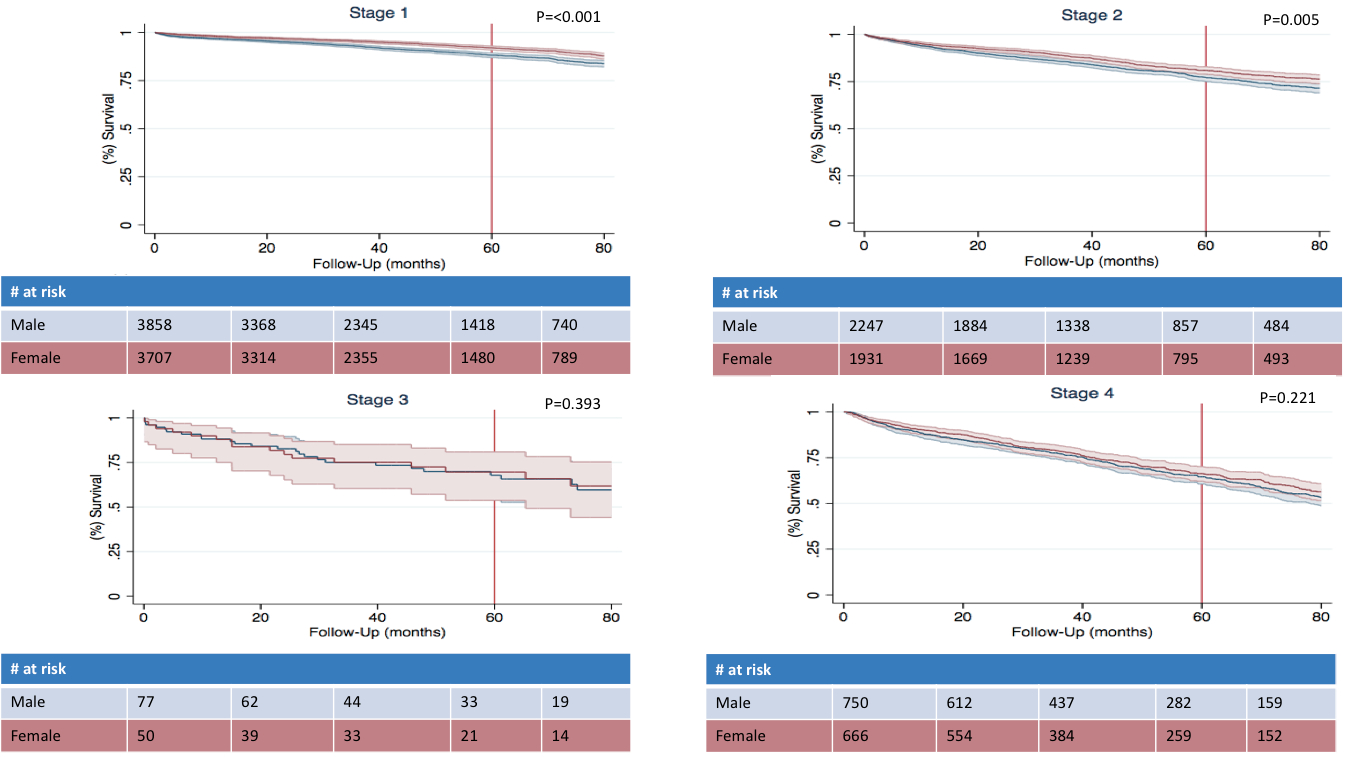
Kaplan–Meier survival analysis by stage of disease and sex
Back to 2022 Abstracts
