|
Back to 2018 Posters
OVERALL AND DISEASE-FREE SURVIVAL IN COLORECTAL CANCER PATIENTS RECEIVING ADJUVANT CHEMOTHERAPY WITH BIOLOGIC AGENT: A SYSTEMATIC REVIEW AND META-ANAYLSIS.
Pablo Serrano Aybar*1, Diana Carter1, Christine Li1, Marlie Valencia1, Leyo Ruo1, Sameer Parpia2, Marko Simunovic1,2, Oren Levine2
1Surgery, McMaster University, Hamilton, ON, Canada; 2Oncology, McMaster University, Hamilton, ON, Canada
Background: Adjuvant therapy for patients undergoing curative resection for stage III and high-risk stage II colorectal cancer improves overall survival (OS). Biologic agents have shown promise as a complement to chemotherapy in the setting of unresectable metastatic colon cancer, however, the effect in stage II and III colon cancer remains unclear.
Methods: Our systematic review and meta-analysis examined the effect of biologic agents when combined with adjuvant chemotherapy for colon cancer. The primary outcome was OS and disease-free survival (DFS). Subgroup analyses were performed based on stage and the type of biologic agents (bevacizumab vs. others). Within the bevacizumab group, an additional subgroup analysis was performed to analyze the effects of microsatellite instability. The intervention group was defined as adjuvant chemotherapy with biologic agents. The control group was defined as adjuvant chemotherapy without biologic agents. We searched MEDLINE, EMBASE and CENTRAL for randomized controlled trials published between January 2002 to February 2017.
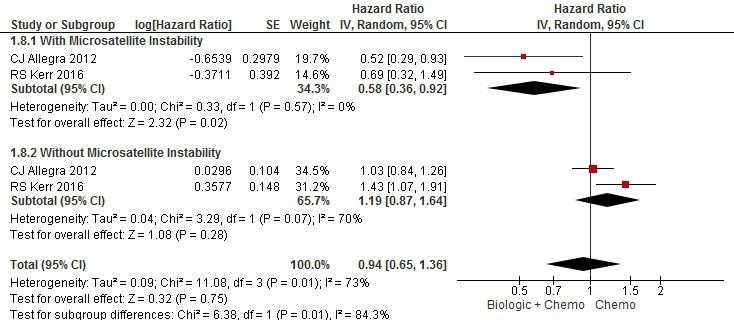
Results: Six trials including 10,754 patients with a median follow-up of 4.8 years were included. In the intervention arm, both OS (HR 2.55, 95% CI 2.37-2.75) and DFS (HR 2.56, 95% CI 2.40-2.73) were significantly worse. Subgroup analyses comparing stage III vs. II and III patients failed to demonstrate a survival benefit in either subgroup; however, explained a degree of heterogeneity between studies. Ultimately, subgroup analysis based on type of biologic agent explained high heterogeneity. With respect to OS and DFS, both bevacizumab and other biologic agent subgroups showed harm; however, within the bevacizumab subgroup, patients with microsatellite instability presented with improved OS (HR 0.58, 95% CI 0.36-0.92) versus without microsatellite instability (HR 1.19, 95% CI 0.87-1.64).
Conclusions: The addition of a biologic agent to adjuvant chemotherapy in the treatment of high risk stage II and III colon cancer is not associated with survival benefit. Patients with microsatellite unstable tumours may benefit from the addition of bevacizumab to adjuvant chemotherapy.
Figure 1 - Overall survival
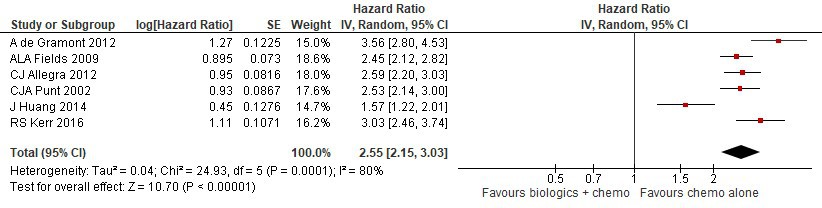
Figure 2 - Meta-analysis of Overall Survival (Subgroup analysis - Microsatellite Instability)
Back to 2018 Posters
|


