|
THE ROLE OF ARYL HYDROCARBON RECEPTOR PATHWAY AFTER SMALL BOWEL RESECTION Emily J. Onufer*1, Kristen Seiler1, David Alvarado2, Misty L. Good3, Matthew A. Ciorba2, Brad W. Warner1 1Surgery, Washington Univeristy in St. Louis, St. Louis, MO; 2Gastroenterology, Washington University in St. Louis, St. Louis, MO; 3Neonatology, Washington University in St. Louis, St. Louis, MO
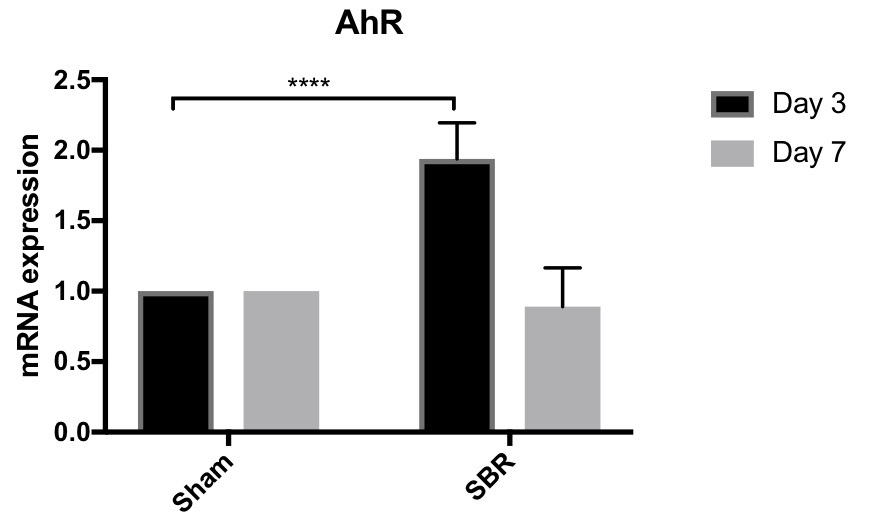
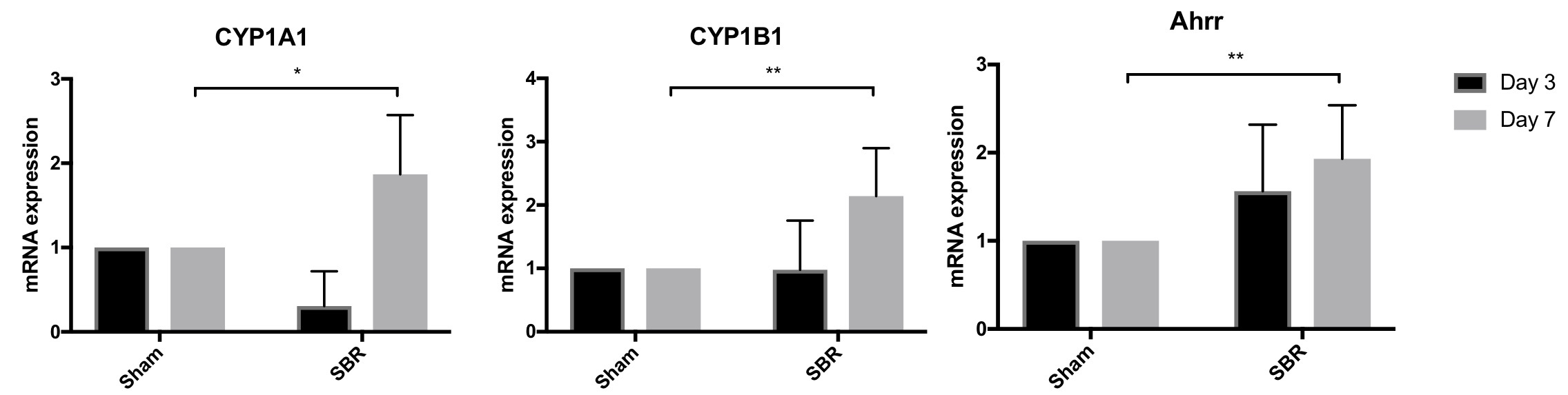
Introduction: Aryl hydrocarbon receptor (AhR) has been shown to play crucial roles in gut homeostasis by modulating inflammatory responses to intraluminal and epithelial stimuli. Pathways downstream of AhR stimulation include expansion of immune populations such as innate lymphoid cells, T-helper 17 cells, and T-regulatory cells, downregulation of Th1 cytokines, and induction of IL-22 expression. Additional studies implicate AhR in tissue regeneration and, furthermore, demonstrate metabolic sequelae of AhR-mediated homeostasis. Following massive small bowel resection (SBR) in mice, there is an adaptive tissue regenerative response to compensate for lost mucosal surface area, which is marked by significant IL-22 upregulation. This is accompanied by metabolic changes (resection-associated metabolic syndrome, or RAMS) including preferential fat (rather than lean body mass) deposition, pancreatic islet cell hyperplasia, and hepatic steatosis. This led us to hypothesize that AhR may be involved in small intestinal adaptation following SBR, potentially mediating RAMS. Back to 2018 Posters |
|||||||||||||||
© 2026 Society for Surgery of the Alimentary Tract. All Rights Reserved. Read the Privacy Policy.