|
INDUCED ENDODERMAL PROGENITOR CELLS ASSUME EPITHELIAL GENE EXPRESSION PROFILES WHEN CULTURED ON ACELLULAR INTESTINAL SCAFFOLDS WITHOUT THE ADDITION OF EXOGENOUS GROWTH FACTORS Kristen Seiler*1, Sarah Waye2,3, William Goo4, Samantha Morris2,3, Brad W. Warner1 1Surgery, Washington University in Saint Louis, Saint Louis, MO; 2Developmental Biology, Washington University, Saint Louis, MO; 3Genetics, Washington University, Saint Louis, MO; 4Washington University, Saint Louis, MO
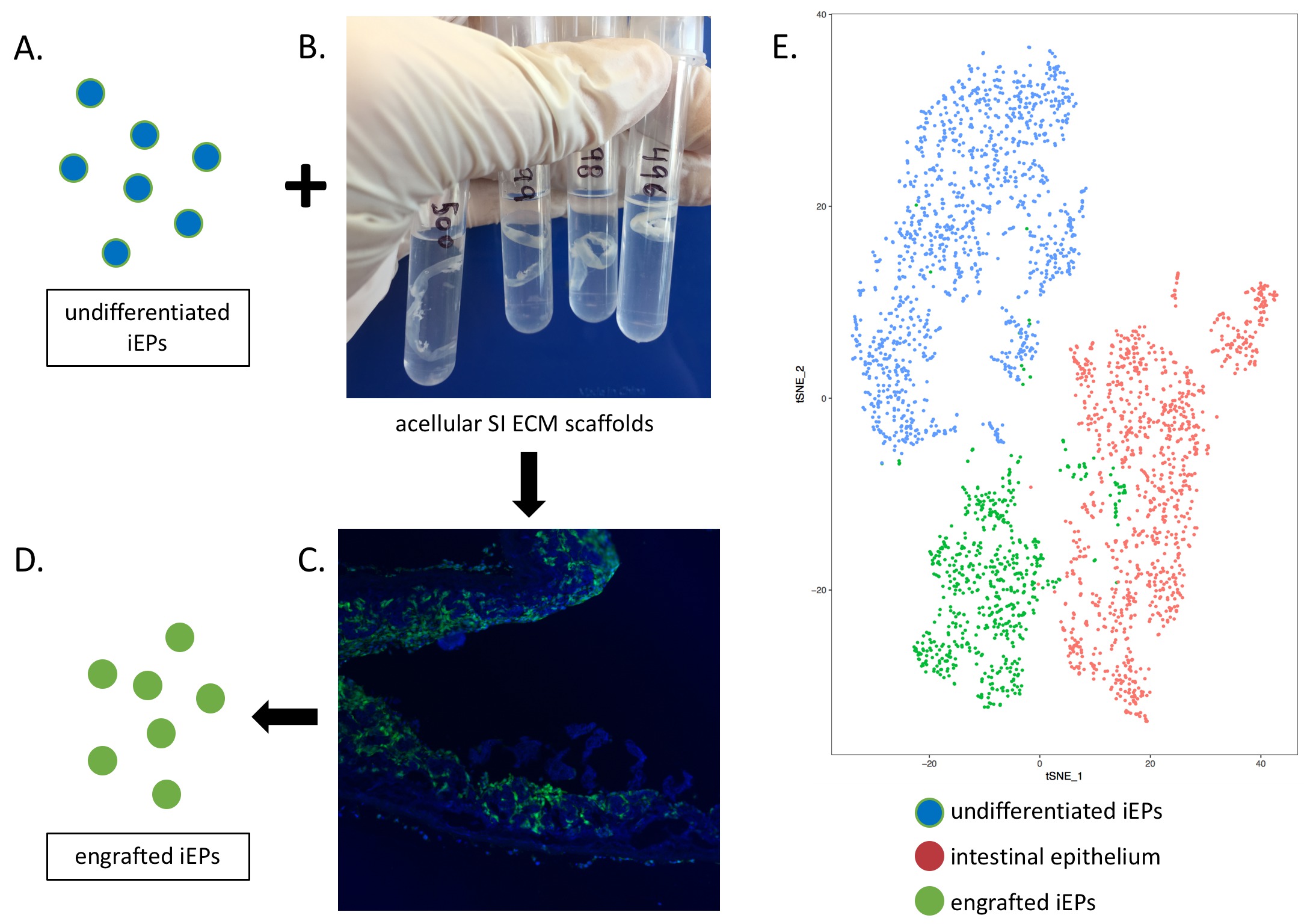
Background: Short gut syndrome (SGS) is a clinically devastating problem that occurs when patients do not have enough small intestine (SI) mucosal absorptive surface area to absorb the nutrients needed to live. Current therapies for SGS are limited to total parenteral nutrition (TPN), intestinal lengthening procedures, and SI transplant, all of which entail significant morbidity and/or mortality. Tissue-engineered small intestine (TESI) is a promising future therapy for SGS, though many advances are needed before this becomes clinical reality. Two advancements needed include: 1) widespread agreement on an appropriate scaffold material for TESI, and; 2) methods to induce lineage differentiation of patient-derived stem cells that, to any extent possible, avoid exogenous growth factors and potentially carcinogenic materials. Extracellular matrix (ECM) is known to play key roles in regenerative and differentiation processes, and has been published as a promising scaffolding material for TESI. Here, we test the hypothesis that SI ECM is sufficient to induce lineage differentiation of induced endodermal progenitor cells (iEPs, derived from fibroblasts) into SI epithelium, without use of exogenous growth factors. Methods: Small intestine from C57/B6 mice was decellularized to generate acellular scaffolds composed of ECM. iEPs (transduced with lentivirus to express GFP) were introduced into the lumen of these scaffolds and cultured in a growth medium devoid of exogenous growth factors. After approximately 2 weeks in culture, iEPs were retrieved from scaffolds and single-cell libraries were prepared using the 10x Genomics Chromium System. t-SNE mapping was employed to visualize clusters of transcriptionally similar cells, as shown in Fig. 1. SI epithelial organoids cultured in a standard Matrigel-based culture system served as a positive control for epithelial gene expression. Results: GFP-expressing iEPs engraft the luminal surface of SI ECM. In the absence of exogenous growth factors, culture on SI ECM induces gene expression changes in iEPs, with lineage differentiation signatures that are largely separate from iEPs, with some partial overlap with SI epithelium. Conclusions: This pilot study indicates transcriptional changes reflecting movement from an iEP identity to a more intestinal epithelial cell fate when cultured on SI ECM. Furthermore, SI ECM is capable of inducing these changes in cell transcriptome in the absence of exogenous growth factors. This builds on an increasing body of evidence that acellular scaffolds are a promising scaffolding substrate for TESI, not only for their ability to support epithelial growth, but also to induce appropriate lineage differentiation of seeded cellular components. Back to 2018 Posters |
|||||||||||||||
© 2026 Society for Surgery of the Alimentary Tract. All Rights Reserved. Read the Privacy Policy.