|
ENDOSCOPIC MANAGEMENT OF RECALCITRANT MARGINAL ULCERS BY COVERING THE ULCER BED. Lea Fayad*1, Sindhu Barola1, Christine Hill2, Thomas Magnuson1, Michael Schweitzer1, Vikesh Singh1, Yen-I Chen1, Saowonee Ngamruengphong1, Mouen A. Khashab1, Anthony N. Kalloo1, Vivek Kumbhari1 1Johns Hopkins Hospital, Baltimore, MD; 2Johns Hopkins Bloomberg School of Public Health,, Baltimore, MD
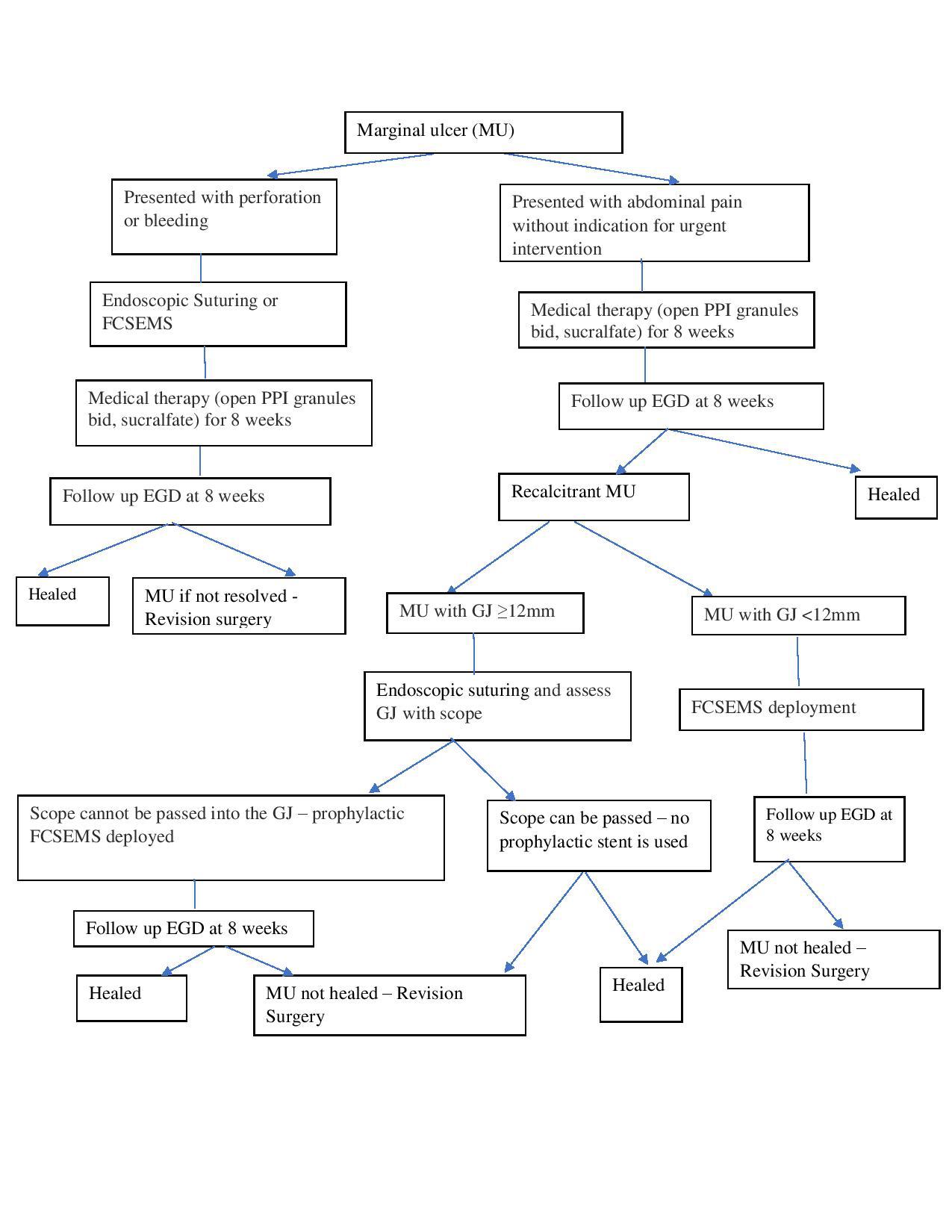
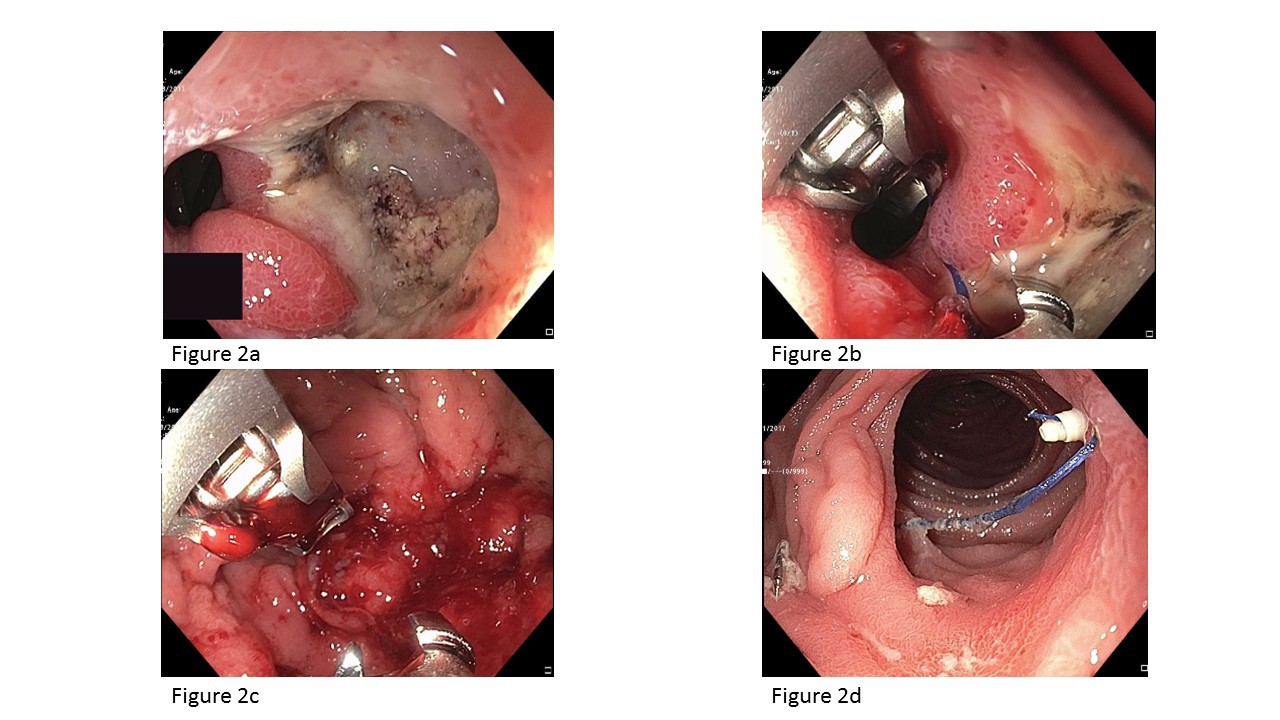
Background: Marginal ulceration (MU) is a commonly encountered adverse event after Roux-en-Y gastric bypass (RYGB). It can be managed medically with smoking cessation and replacement of nonsteroidal anti-inflammatory drugs (NSAIDs) with non-ulcerogenic analgesics,use of proton pump inhibitors (PPIs) and the endoscopic removal of staples/suture material.Marginal ulcers refractory to medical therapy may require revision surgery, which is associated with high morbidity and possible recurrence. Endoscopic management may be a suitable alternative to revision surgery. Scattered reports have achieved success with oversewing using an endoscopic suturing system, but the mechanism is unclear and may be related to covering the ulcer bed. By the same rationale, using fully covered self-expandable metallic stents (FCSEMS) to cover the ulcer bed may also be used to treat MUs in smaller gastric outlets that are not suitable for oversewing. Aims: To evaluate technical feasibility, efficacy, and safety of endoscopic management of MU by covering the ulcer bed using oversewing and/or deploying a FCSEMS using a predetermined algorithm (Figure 1). Methods: Medical records of consecutive patients who underwent endoscopic suturing and/or FCSEMS deployment for recalcitrant MU between August 2016 and June 2017 at a single academic center were reviewed. Recalcitrant MU was defined as an ulcer that persists after 6 to 8 weeks despite maximal medical therapy, cessation of smoking and NSAIDS, and H. pylori eradication. Suturing (Figure 2) was performed using a single suturing technique or a double suturing technique when there were ≥ 2MUs. A FCSEMS was used when the gastric outlet was significantly reduced in size by oversewing or the marginal ulcer was in the context of stenosis of the gastric outlet. Results: Eleven patients (age 31-60; all females) with mean BMI of 27.72 ± 5.93kg/m2 underwent endoscopic suturing and/or stent deployment for recalcitrant MU at a median of 50 months (range 1.3-84)post-RYGB. The indications for endoscopic therapy were acute perforated MU (n=2) and pain due to a recalcitrant MU (n=9). Seven patients were managed by oversewing, two were managed by FCSEMS, and two patients required both. Technical success was 100 percent. Transient abdominal discomfort was noted within the first 24 hours of the procedure only and all patients reported resolution of abdominal pain at one week. Patients remained asymptomatic on further clinical follow-up at 3 monthly intervals for 1 year. Surveillance endoscopy performed in 10/11(90.9%) patients at 8 weeks revealed complete ulcer healing in 9/10 (90%). There were no adverse events or stent migration. Conclusion: Endoscopic management is a technically feasible,effective and safe method to treat recalcitrant MU and should be considered an alternative to surgical revision. It also appears effective for perforated MU. Back to 2018 Posters |
|||||||||||||||
© 2026 Society for Surgery of the Alimentary Tract. All Rights Reserved. Read the Privacy Policy.