|
Back to 2018 Program and Abstracts
SELECTIVE HISTONE ACETYLTRANSFERASE INHIBITORS DECREASE GROWTH AND INDUCE APOPTOSIS IN PANCREATIC ADENOCARCINOMA
Saba Kurtom*1,2, Bhuwan Giri1, Vrishketan Sethi1, Bharti Garg1, Anthony Ferrantella1, Harrys Jacob1, Sulagna Banerjee1, Sundaram Ramakrishnan1, Ashok Saluja1, Vikas Dudeja1
1Surgery, University of Miami, Miami, FL; 2Surgery, Virginia Commonwealth University, Richmond, VA
Background:
Recent focus on epigenetic changes in cancer has demonstrated that post-translational modification of histones including acetylation and methylation regulates cancer growth. Histone acetyltransferases (HATs) are enzymes that acetylate histones, leading to activation of gene transcription. The aim of our study is to evaluate the role of HAT inhibitors as a potential therapy against pancreatic ductal adenocarcinoma (PDAC).
Methods:
HATs were inhibited in vitro in pancreatic cancer cell lines S2VP10 and Mia PaCa-2 by C646, a selective HAT inhibitor. Cell viability and apoptosis were measured by CCK-8 assay and Caspase Glo 3/7 assay, respectively. Cell cycle analysis was performed using flow cytometry. Histone acetylation was analyzed by western blotting. In vivo experiment involved implantation of subcutaneous tumors (Mia-PaCa-2 cells) into athymic nude mice. Mice were randomized to control or treatment group (C646 daily).
Results:
C646 at a dose range of 20-100 µM resulted in a dose and time dependent cytotoxicity in Mia PaCa-2 and S2VP10 cells with corresponding increase in caspase-3 cleavage. C646 selectively decreased acetylated levels of Histone 3 and Histone 4. A G2/M Phase arrest was observed in cells being treated with C646 at a dose of 25 µM. In vivo experiments on athymic nude mice revealed a significant decrease in subcutaneous tumor volume progression and tumor weight in C646-treated mice when compared to the control group.
Conclusion:
Histone acetyltransferase inhibition leads to pancreatic cancer cell death. HAT inhibitors have the potential to become part of novel treatment strategies for pancreatic cancer.
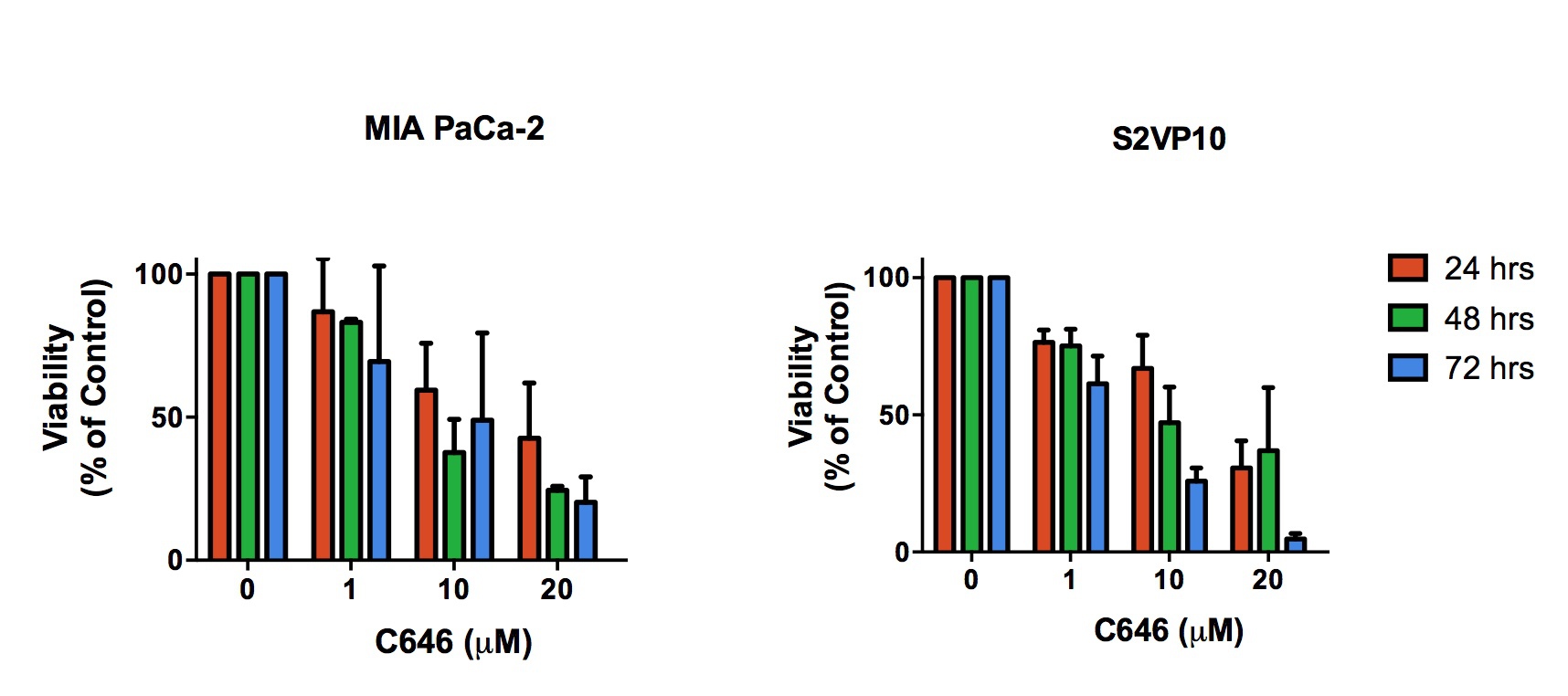
C646 treatment induces dose-dependent cell death in pancreatic cancer cells.
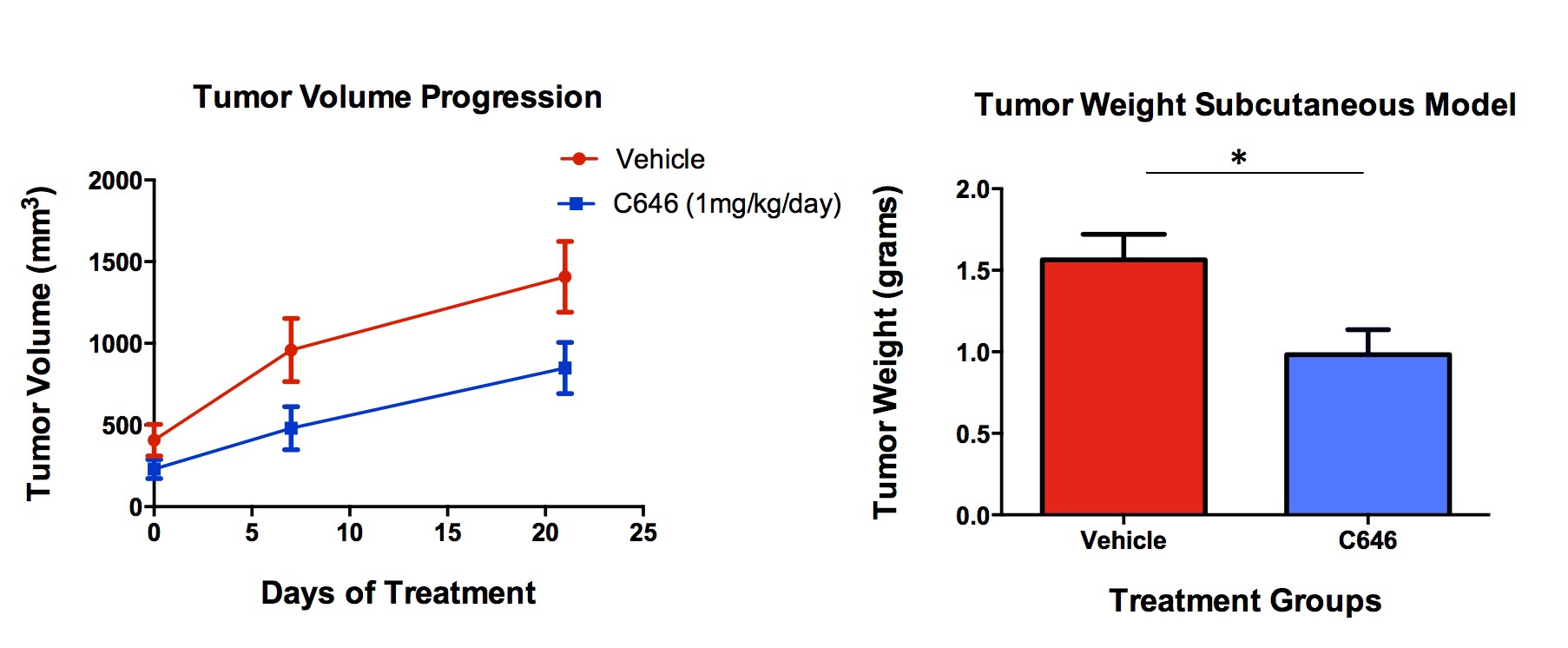
C646 delays tumor progression in subcutaneous xenograft model of PDAC
Back to 2018 Program and Abstracts
|


