|
Back to 2018 Program and Abstracts
PANITUMUMAB-IRDYE800CW FOR FLUORESCENCE-GUIDED SURGICAL RESECTION OF COLORECTAL CANCER.
John C. Marston*1, Yolanda E. Hartman2, Jason M. Warram2,4, Gregory D. Kennedy3
1University of Alabama School of Medicine, Birmingham, AL; 2Otoloaryngology, University of Alabama at Birmingham, Birmingham, AL; 3Surgery, University of Alabama at Birmingham, Birmingham, AL; 4Radiology, University of Alabama at Birmingham, Birmingham, AL
Background The staging and risk stratification of malignant colon polyps is difficult and often imprecise. A particularly troublesome task is assessing the risk of lymphatic spread. There is room for improvement in the current strategy, which uses histologic scoring systems to help guide clinicians on the proper resection method and follow up timeline. Fluorescence-guided surgery (FGS) utilizes fluorescently-tagged antibodies and specialized imaging devices to characterize tumors beyond the capabilities of conventional imaging. Application of FGS technology to colorectal cancer will allow for detailed assessment of tumor spread and more accurate risk stratification of malignant colon polyps.
Aim To evaluate a panitumumab-IRDye800CW conjugate as an optical imaging agent in the application of FGS technology to colorectal cancer.
Methods Three colorectal adenocarcinoma cell lines (SW948, Colo205, and LS174T) were probed for epidermal growth factor receptor expression and implanted subcutaneously into athymic nude mice. Panitumumab, an epidermal growth factor receptor inhibitor, was conjugated to IRDye800CW, a near infrared dye frequently used in FGS. After 5mm tumors grew, half the mice were injected with 200mcg of panitumumab-IRDye800CW. The remaining mice received 200mcg of IgG-IRDye800CW as a control agent. Fluorescence imaging was performed each day for 10 days with closed and open-field devices. On day 10 the mice were sacrificed and their tumors were resected, imaged, and sectioned for immunohistochemical and hematoxylin and eosin staining. Tumor to background ratio (TBR) was calculated for each day of imaging by dividing the mean fluorescence intensity (expressed in pixels) within the tumor by the mean fluoresecence intensity in healthy tissue.
Results The panitumumab-IRDye800CW group had significantly greater TBR values in all cells lines for each day of imaging.
Conclusion These data demonstrate the superiority of panitumumab-IRDye800CW as an optical imaging agent in the application of FGS technology to colorectal cancer, and warrant further investigation into the role of FGS in its staging and treatment. Hematoxylin and eosin and immunohistochemical staining of tumor sections is forthcoming. This will allow for assessment of panitumumab-IRDye800CW specificity for colorectal cancer cells and any areas of false positive or false negative fluorescence. Possible areas of future interest include imaging resected human colorectal cancer specimens for lymphatic spread after a preoperative dose of panitumumab-IRDye800CW.
Results
| | TBR | LS174T | Colo205 | SW948 | | Panitumumab-IRDye800CW | Day1 | 2.05 ± 0.255 | 1.23 ± 0.176 | 3.87 ± 0.454 | | | Day 10 | 10.9 ± 2.94 | 5.22 ± 1.66 | 3.19 ± 1.71 | | IgG-IRDye800CW | Day 1 | 1.20 ± 0.311 | 0.895 ± 0.131 | 1.63 ± 0.321 | | | Day 10 | 4.15 ± 1.39 | 1.03 ± 0.048 | 2.35 ± 0.321 | | | | | | | | P-value (all cell lines) | Day 1 | 0.22 | Day 10 | 0.18 |
Average TBR for each cell line on days 1 and 10 of imaging with closed field device. 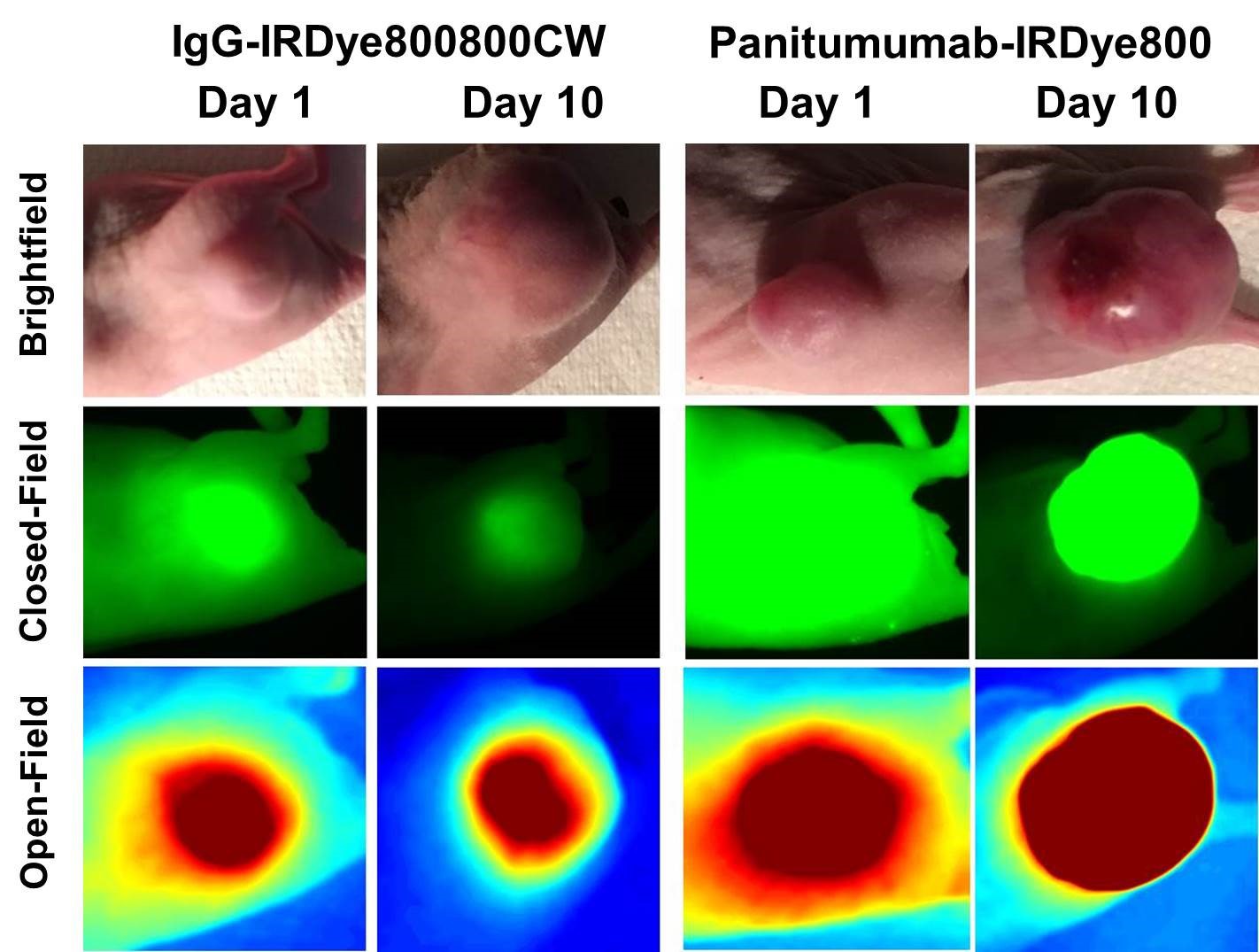 Mice in the panitumumab-IRDye800CW group had significantly greater TBR values qualitatively and quantitatively with both imaging modalities.
Back to 2018 Program and Abstracts
|

