
|
 |
Back to 2018 Program and Abstracts
IMPACT OF SINGLE FLUID-FILLED INTRAGASTRIC BALLOON ON METABOLIC PARAMETERS AND NONALCOHOLIC STEATOHEPATITIS: A PROSPECTIVE PAIRED ENDOSCOPIC ULTRASOUND GUIDED CORE LIVER BIOPSY AT THE TIME OF BALLOON PLACEMENT AND REMOVAL
Fateh Bazerbachi1, Eric J. Vargas1, Taofic Mounajjed2, Sudhakar K. Venkatesh3, Kymberly D. Watt1, John D. Port3, Rita Basu6, Monika Rizk1, Andres Acosta1, Ibrahim Hanouneh5, Naveen Gara4, Meera Shah7, Manpreet Mundi7, Matthew Clark8, Karen Grothe8, Andrew C. Storm1, Mark Topazian1, Michael J. Levy1, Christopher Gostout1, Barham K. Abu Dayyeh1
1. Gastroenterology, Mayo Clinic, Winnipeg, MB, Canada; 2. Pathology , Mayo Clinic, Rochester , MN; 3. Radiology, Mayo Clinic, Rochester , MN; 4. Gastroenterology, UCSD, San Diego, CA; 5. Minnesota Gastroenterology, Minneapolis, MN; 6. Endocrinology, University of Virginia, Charlottesville , VA; 7. Endocrinology, Mayo Clinic, Rochester, MN; 8. Psychiatry, Mayo Clinic, Rochester, MN
INTRODUCTION
Nonalcoholic Steatohepatitis (NASH) with early fibrosis is serious condition that afflicts a significant portion of patients with obesity with the potential to progress to cirrhosis. The degree of weight loss is independently associated with improvements in all NASH-related histologic parameters when a threshold of ≥ 10% of total body weight (%TBWL) is achieved. Only the minority of patients can reach this threshold with non-surgical obesity interventions. The Orbera Intragastric Balloon (IGB) (Apollo Endosurgery, Austin, TX) is a safe endoscopic bariatric device that achieves ≥ 10% TBWL in the majority of patients . We aimed to prospectively evaluate the impact of this IGB on metabolic and histologic NASH parameters.
METHODS
This is prospective open-label study combining a paired endoscopic ultrasound-guided core liver biopsy (EUS-LB) at the time of Orbera™ IGB placement and removal. All patients had NASH with early fibrosis diagnosed by Magnetic Resonance Elastography (MRE) and the MRE was repeated after balloon removal. This study was IRB and FDA approved. All biopsies and MREs were evaluated by an experienced liver pathologist and radiologist, respectively, both blinded to the clinical status and data of the patient.
RESULTS
Baseline characteristics are shown in table 1. At time of balloon removal patients lost an average of 12.8 ± 5.3% TBWL. This was associated with significant improvement in their metabolic profile with decrease in HbA1c (7.5±0.4 to 6.3±0.3; p=0.004) and central obesity (waist circumference decreased by 8.6±13 cm; p=0.02). Radiologic metabolic components and liver stiffness improved significantly on repeat MRE (Figure 1). NASH activity score (NAS), on liver biopsy, improved in 87% of patients with a median decrease of 3 points (range: 1, 4). Seventy three percent of patient achieved ≥ 2 points improvement in NAS. Overall fibrosis regressed in 20% of patients. All patients with early fibrosis (stages 1A, 1B, and 1C) regressed. AST and AST to platelet ratio (APRI score), respectively, improved significantly (75.15±13 vs. 25±5; p=0.001; 1.5±0.4 vs. 0.6±0.2; p=0.001). EUS-LB related adverse events occurred in 5% of procedures, and were restricted to post-procedural pain without bleeding. One patient stopped their use of antithrombotics against medical recommendations, and suffered from a coronary event 1 month after IGB placement.
CONCLUSION
This study provide conclusive evidence that weight loss achieved with the single-fluid intragastric balloon is associated with significant metabolic improvement and regression of NASH; thus providing a safe and effective tool to manage this disease when combined with weight maintenance strategy after balloon removal.
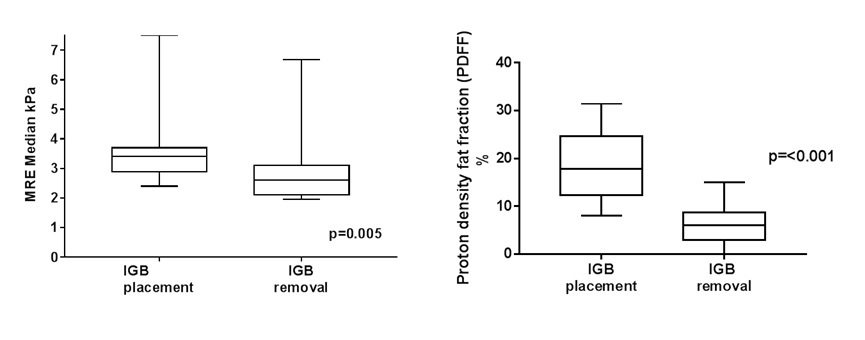
Table 1. Baseline characteristics
| Total patients/dropped patients | 21/1 | | Gender, female | 17 (85%) | | Age (median [range]) | 53 [34, 65] | | Waist circumference (median [range]) | 44 [32, 55] | | Hip circumference (cm) (median [range]) | 129 [106 , 155] | | Waist-hip ratio in men (median [range]) | 131 [107,174] | | Waist-hip ratio in women (median [range]) | 0.94 [0.81 , 1.04] | | Fibrosis | | | 1A | 6 (28%) | | 1B | 1 (5%) | | 1C | 2 (10%) | | 2 | 7 (33%) | | 3 | 5 (24%) | | NAS score (median [range]) | 4 [2, 5] | | Liver stiffness (kPa) (median [range]) | 3.3 (2.4, 7.5) | | %Total body weight loss (median [range]) | 12 (0, 32.5) | | %TBWL (mean± standard deviation) | 12.8 ± 5.3 | | | |
Back to 2018 Program and Abstracts
|

