
|
 |
Back to 2018 Program and Abstracts
CANCER VACCINE TARGETING MYB IN COLORECTAL CANCER: FROM PRE-CLINICAL MODEL TO CLINICAL TRIAL
Toan Pham*1,2, Shienny Sampurno1, Lloyd Pereira1, Sara Roth1, Vignesh Narasimhan1,2, Sandra Carpinteri1, Alexander Heriot2, Jayesh Desai3, Robert G. Ramsay1
1Cancer Research, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; 2Cancer Surgery, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; 3Oncology, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Background
MYB, a transcription factor, is overexpressed (o/e) in >80% of colorectal cancer. MYB o/e correlates with lower T-cell infiltrate and poorer prognosis. Patients with MYB o/e are therefore the ideal target for cancer vaccines that increase recruitment of tumour infiltrating lymphocytes. A single institution has engineered a DNA vaccine against MYB; TetMYB.
Published pre-clinical data has shown remarkable efficacy against MC38 mouse colorectal cancer; a C57BL/6 model. This vaccine induces tumour regression and protective immune memory against further tumour rechallenge. These promising data have led to a first-in-human phase I/II clinical trial, MYPHISMO (NCT03287427).
We also conducted studies of prophylactic and therapeutic applications against colonic adenoma.
Methods
MYPHISMO Trial
This single-arm, multi-centre clinical trial in patients with Stage IV colorectal cancer, evaluates the safety profile of TetMYB as a monotherapy and in combination with a PD-1 inhibitor (phase I), and assesses the efficacy of the combination therapy in a larger cohort (phase II). The target sample size is 32 patients: 12 in phase Ia and 20 in phase II. The expected trial duration is 3 years with 18 months follow-up.
TetMYB in Colonic Adenoma Model
Colonic adenoma was induced in APC580S/+Villin-Cre-ERT2 model with tamoxifen enriched feed for 24 hours.
Prophylactic Study: 15 mice
Treatment arm (8 mice): 3 vaccinations using TetMyb at weeks 7, 9 and 11 followed by tamoxifen induction at week 13.
Control arm (7 mice): tamoxifen induction at week 13.
Mice were culled upon reaching ethical endpoints.
Therapeutic Pilot Study: 7 mice
Tamoxifen induction at week 9 followed by weekly colonoscopy. Upon detecting adenoma, weekly vaccinations with TetMyb (6 doses) & anti-PD1 antibody (4 doses). Adenoma progression quantitatively assessed with adenoma area to lumen ratio.
Endpoint is bowel obstruction confirmed on colonoscopy.
Results
TetMYB in Pre-Clinical Colonic Adenoma Model
Prophylactic study: median survival of TetMyb cohort was 356 days vs 183 days of the control group (p = 0.0118). Hazard ratio of control vs TetMyb was 3.087 (95% CI: 0.9114-10.46).
Therapeutic pilot study: all mice alive at 235 days. Adenoma detected in 5/7 mice. Median time to adenoma detection was 155 days. All mice with adenoma completed all treatment. One mouse developed a large colonic adenoma whose regression after intervention, over a period of 62 days, was statistically significant (p = 0.0002).
Conclusion
Our pre-clinical data suggests TetMYB vaccine is protective against colorectal carcinoma. The MYPHISMO clinical trial will provide safety and early efficacy data to allow progression to a phase III trial.
Our pre-clinical data suggests TetMYB also has anti-adenoma activity. This result will be validated in a larger scale experiment before progression to clinical trial.
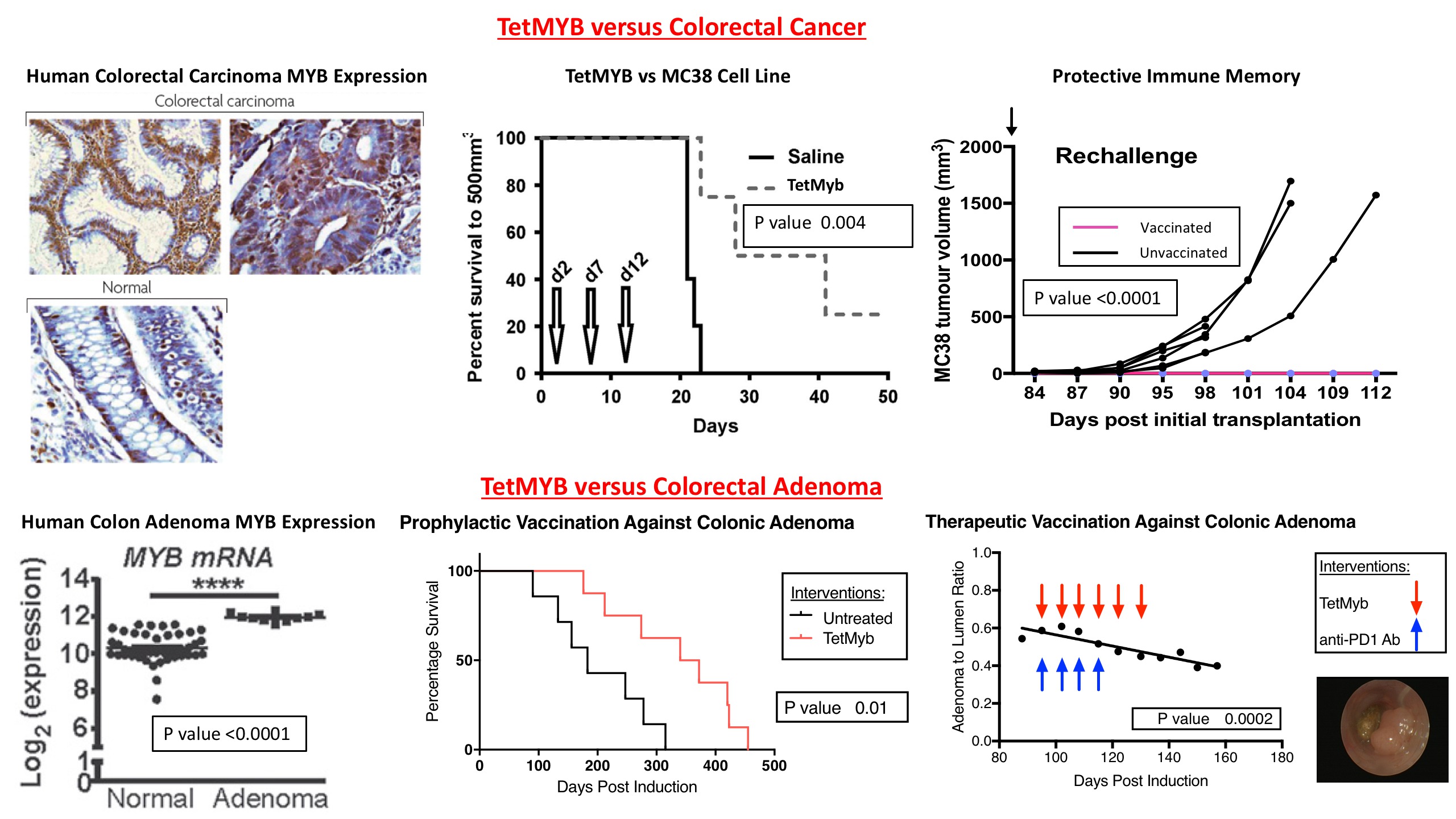
Pre-clinical Data of TetMYB versus Colorectal Carcinoma (top row) and Adenoma (bottom row).
Back to 2018 Program and Abstracts
|

