
|
Back to 2018 Program and Abstracts SURGICAL AND PATIENT-CENTERED OUTCOMES AFTER PANCREATIC RESECTION IN HIGH-RISK INDIVIDUALS FOR PANCREATIC CANCER: A 20-YEAR PROSPECTIVE CANCER OF THE PANCREAS SCREENING (CAPS) COHORT STUDY Tossapol Kerdsirichairat*1, Marcia I. Canto1, Madeline Ford1, Michael G. Goggins1, Amanda Blackford3, Toshiya Abe1, Ralph Hruban2, Anne Marie Lennon1, Alison Klein3, Ihab R. Kamel4, Elliot K. Fishman4, Matthew J. Weiss5, Charles J. Yeo5, Richard D. Schulick6, Christopher L. Wolfgang5 1Gastroenterology/Medicine, Johns Hopkins University, Baltimore, MD; 2Pathology, Johns Hopkins University, Baltimore, MD; 3Biostatistics and Bioinformatics, Johns Hopkins University, Baltimore, MD; 4Radiology, Johns Hopkins University, Baltimore, MD; 5Surgery, Thomas Jefferson University, Philadelphia, PA; 6Gastroenterology/Medicine, University of Colorado, Denver, CO
Screening high-risk individuals (HRI) for pancreatic adenocarcinoma (PDAC) and its precursors is a potential approach to improving survival. However, little is known about the outcomes after pancreatic resection for screening-detected pancreatic lesions. We report postoperative surgical and patient-centered outcomes. METHODS: HRI enrolled in prospective CAPS surveillance studies (1998-2017) consisted of relatives from familial PC kindreds, those with Peutz-Jeghers syndrome, germline BRCA1/2 mutations, PALB2, p16/CDKN2A, ATM, or Lynch syndrome. Those who had surgery for pancreatic lesions detected by EUS/MRI/CT and >6 months follow-up were included in this analysis. Surgical outcomes (mortality, morbidity, length of stay, readmission, diabetes), patient-outcomes (pain, patient satisfaction (Likert scale of 1 completely satisfied to 7 completely dissatisfied), pre- and post-operative health-related quality of life (QoL, Short Form 36) were assessed. Survival was estimated using Kaplan-Meier analysis. RESULTS: Among 354 HRI with continued surveillance, 46 (13%) asymptomatic HRI had surgery for suspected pancreatic neoplasms. 10/11 (91%) screening-detected PDACs were resectable (all R0) (by comparison rates of resection of PDAC in the US are ~20%) with 2(18%) Stage IA (18%), 2(18%) Stage IIA, 6(54%) Stage IIB, and 1(9%) Stage IV(Table 1). 40 HRI had partial resection, then 8 of these underwent completion pancreatectomy at median 3.81 years (IQR 2.5-7.6) for a new lesion. 2/8 (25%) of the HRI with partial resection developed PDAC in the remnant pancreas. Postoperative complications developed in 15 HRI (32.6%): delayed gastric emptying (6 Whipple (W), 1 distal pancreatectomy (DP), 2 total pancreatectomy (TP)), surgical site infection (2 W, 2 DP), grade B pancreatic fistula (2 W, 2 DP) and cholangitis (2 W, 1 DP). There were no 90-day perioperative deaths or hemorrhage. Median length of stay was 7 days (IQR 5-11), with 3 30-day readmissions. Diabetes developed in 6/28 HRI with no prior diabetes after partial resections. 11(24%) use an insulin pump, with 2 ER visits (1 admission in a post TP patient) related to hypo/hyperglycemia. Median follow-up was 7.3 years, IQR 4.2-9.9). There were 8 deaths, 5 related to PDAC (10.9%). 3-year and 5-year overall survival were 97.7% and 88.2%, respectively. Preoperative and postoperative median physical component scores (90 vs. 95, respectively, p=0.44) and mental component scores (84 vs 88, respectively,0.81) were comparable. Median postoperative bodily pain score was 90, (baseline 90, p=0.32). Median post-operative patient satisfaction was 2 (IQR 2-3). CONCLUSION: Screening HRI leads to detection of PDAC, improved resectability, and stage shift compared to known national rates. Surgical resection is associated with acceptable morbidity and no mortality in this series. Patient satisfaction is high and QoL is preserved.
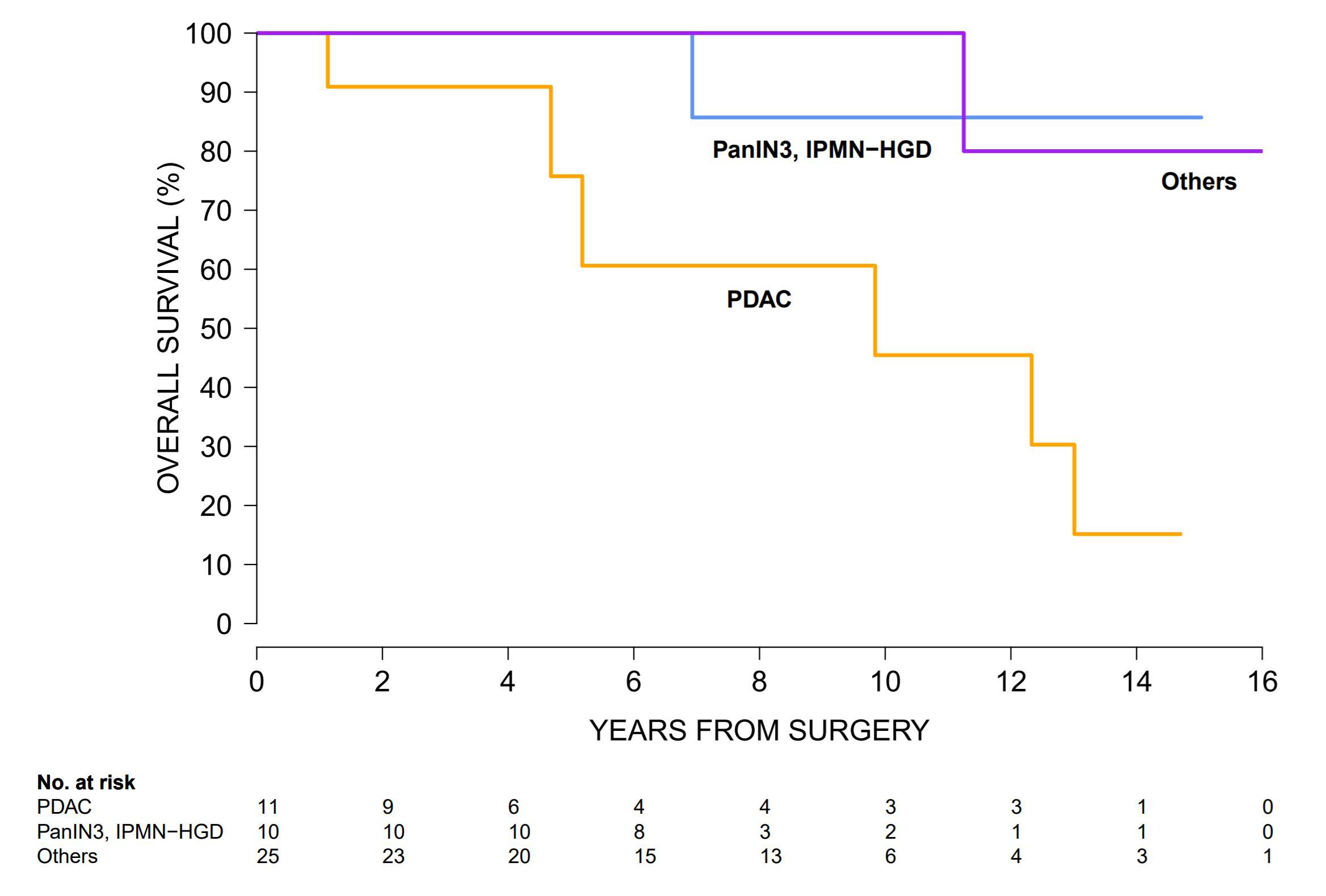 Figure 1: Survival of high risk individuals (HRI) after pancreatic resection using Kaplan-Meier's method and compared using the log-rank test. Comparing to those with pancreatic ductal adenocarcinoma (orange), those with high-grade dysplastic precursors (blue) had comparable survival (p=0.14) and those with other low-grade dysplastic precursors (purple) had better overall survival (p=0.02). Back to 2018 Program and Abstracts |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© 2026 Society for Surgery of the Alimentary Tract. All Rights Reserved. Read the Privacy Policy.