
|
 |
Back to 2018 Program and Abstracts
PREOPERATIVE INTESTINAL MICROBIAL DYSBIOSIS IS ASSOCIATED WITH POSTOPERATIVE ILEUS IN PATIENTS UNDERGOING COLORECTAL SURGERY
Benjamin D. Shogan*1, Emilie Duchalais2, Danielle Collins2, Melissa Chang3, Kimberly R. Krull2, David W. Larson2, Marina Walther-Antonio2, Nicholas Chia2, Jun Chen2, Heidi Nelson2
1Colon and Rectal Surgery, University of Chicago, Chicago, IL; 2Colon and Rectal Surgery, Mayo Clinic, Rochester, MN; 3Saint Joseph Mercy Health System, Ann Arbor, MI
Background: Despite significant improvements in patient selection and utilization of enhanced recovery protocols, surgical complications remain a major concern. Emerging evidence suggest a clear role for the microbiome in the development of surgical site infections (SSI) and anastomotic leak. Here we hypothesize that microbial dysbiosis (alterations in the microbiome) may predict patient outcomes following colorectal surgery.
Methods: Over a 10-month period, 101 patients (aged >18 years) undergoing segmental or total colorectal surgery were included in the study. Preoperative microbial samples were collected from the rectum and abdominal skin in the operating room (POD0) and in the hospital on postoperative day 2 (POD2). After quality control, 334 microbial samples were analyzed. Bacterial DNA was extracted, and the V3-V5 region of the 16S rRNA gene was amplified and all samples sequenced by MiSeq simultaneously. Paired-end sequence reads were processed via Hybrid-denovo bioinformatics pipeline. Clinical variables were abstracted by chart review and patient interview. Ileus was defined as >2 episodes of vomiting, or the inability to tolerate diet and/or absence of flatus >24 hours, that occurred on or after the 4th postoperative day (Vather, J GI Surg, 2013).
Results: While we found no significant changes in either the skin or rectal bacterial communities (POD0 or POD2) in patients with a postoperative SSI, anastomotic leak, reoperation, or whom had a 30-day readmission, there were significant differences in bacterial communities (POD0 and POD2) in patients who developed postoperative ileus (POI). While certain variables (diagnosis of ulcerative colitis or malignancy, and the preoperative exposure to antibiotics, bowel prep, or chemoradiation) were found to have independent effects on the microbiome, when these effects were controlled for, the changes in the microbiota associated with patients developing POI remained significant. In patients who experienced ileus, bacterial communities recovered from the rectum on POD0 showed compositional changes (PERMNOVA, p=.004; fig 1A) and increased α-diversity (Linear regression, p=0.02; sfig 1B). Differential abundance analysis of POD0 samples revealed increased amounts of the genus Bacteroides, Parabacteroides, and Ruminococcus in those developing POI (fold change: 2.03, 2.82 and 3.57 respectively; Permutation test, FDR<0.05, fig 1C). These microbiota changes observed in POD0 persisted on POD2. POI was associated with an increased length of stay (5 days+/-SD 4.1 vs 9.1+/-3.0; p=.002).
Conclusion: Alterations of the preoperative intestinal microbiome appears to be a risk factor for the development of postoperative ileus, potentially implicating ileus as a microbial disease. This novel finding may significantly improve our ability to identify and manage patients at risk for developing postoperative ileus.
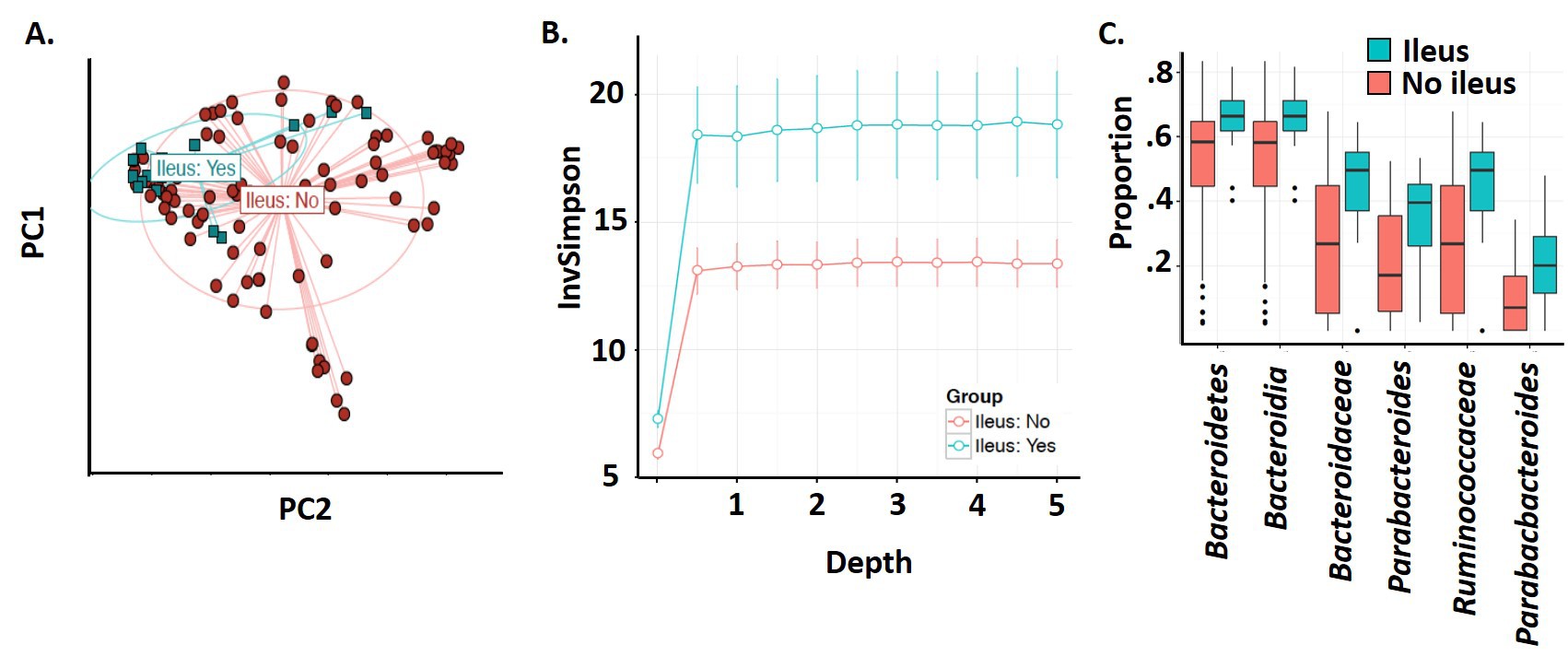
Figure 1. The microbiota recovered on POD0 in patients whom develop postoperative ileus is significantly different in β-diversity analysis (A), α-diversity analysis (B), and differential abundance analysis (C).
Back to 2018 Program and Abstracts
|

