|
Back to 2017 Posters
NOD2 ALLELE VARIANT AND MICROBIOME DIFFERENCES CONTRIBUTE TO THE OCCURRENCE OF POUCHITIS IN ONE OF TWO FAP SIBLINGS FOLLOWING ILEAL POUCH-ANAL ANASTOMOSIS
Kathleen M. Schieffer*1, Justin Wright2, Leonard Harris1, Sue Deiling1, Gregory S. Yochum1,3, Regina Lamendella2, Walter Koltun1
1Surgery, Pennsylvania State University College of Medicine, Hershey, PA; 2Juniata College, Huntingdon, PA; 3Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA
Introduction
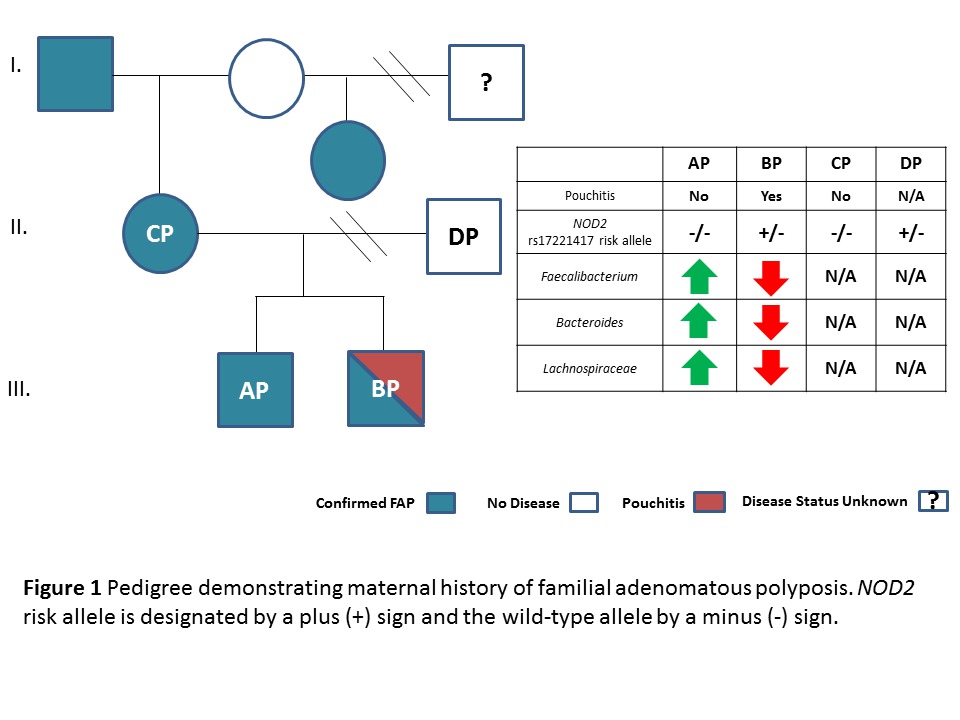
Total proctocolectomy with ileal pouch-anal anastomosis (IPAA) is a common surgical method for treatment of familial adenomatous polyposis (FAP) and ulcerative colitis (UC). Inflammation of the ileal pouch reservoir, termed pouchitis, is common in UC patients but rare in FAP. This differential disease outcome is thought to be related to both microbial and genetic factors. In 2009, due to a family history of FAP, siblings AP (18 yo) and BP (16 yo) underwent IPAA surgery. Patient BP developed severe pouchitis within 3 months of surgery and has intermittently since, while patient AP has remained pouchitis-free. The mother, CP, also had IPAA surgery and has not developed pouchitis. A remote family history of Crohn’s disease was identified, so we investigated this pedigree to elucidate the possible genetic and microbial differences that might explain this unusual picture of pouchitis in a single FAP patient.
Methods
The family pedigree is shown in Figure 1. The two brothers (AP and BP) were investigated, in addition to their parents (CP and DP). Due to its role in innate mucosal host defense in recognizing bacterial antigens, SNPs in the NOD2 gene [rs2076756, rs17221417, rs2066844, rs2066847] were interrogated using a custom Illumina array. Risk allele variants identified were subsequently confirmed by Sanger sequencing. The composition of mucosa-associated bacteria was defined using 16S rRNA gene sequencing on terminal ileum and rectal tissue obtained immediately after surgical resection from the two brothers (AP and BP) using the Illumina MiSeq and analyzed using QIIME 1.9.0.
Results
The sibling with pouchitis (BP) carried a single CD-associated risk allele for NOD2 (rs17221417 G), whereas his brother and mother did not. Microbiome sequencing found reduced levels of what has been suggested to be in other studies “beneficial” bacteria (Faecalibacterium prausnitzii, Bacteroides, and Ruminococcaceae) in BP relative to his brother, AP.
Conclusion
These findings implicate the NOD2 signaling pathway in the pathogenesis of pouchitis. Differences found in the microbiome of pouchitis patients may be due to alterations of host innate immunity, including the NOD2 pathway.
Back to 2017 Posters
|


