|
Back to 2017 Posters
MONITORING OF GASTRIC MYOELECTRIC ACTIVITY AFTER PANCREATICODUODENECTOMY
Monica M. Dua*1, Lavina Malhotra1, Anand R. Navalgund2, Steve Axelrod2, George Triadafilopoulos3, Brendan Visser1
1Surgery, Stanford University, Stanford, CA; 2G Tech Medical, Mountain View, CA; 3Medicine, Stanford University, Stanford, CA
Background: Delayed gastric emptying (DGE) is a common and frustrating complication of pancreaticoduodenectomy (PD). Postoperative DGE may require prolonged nasogastric decompression which causes patient discomfort, increases risk for aspiration events, and prolongs hospital stay. Since the pathophysiology of DGE is unclear, there is no way to predict in which patients DGE is likely to occur. The purpose of this study is to assess whether continuous monitoring of postoperative gastrointestinal motor activity after PD is reproducible and can identify patients that may be at risk for developing DGE.
Methods: Thirty patients were enrolled in this trial between April and November 2016. After PD, three battery-operated wireless patches that acquire myoelectrical signals from the stomach, small intestine, and colon were placed on the anterior abdomen immediately following surgery. The system transmits signal data by Bluetooth to a programmed iPod Touch that also has a user interface that allows the patient to enter clinical parameters. Post-processing of data included removal of large amplitude artifacts and band-pass filtering, followed by Fourier transformation to frequency space over selected time subintervals. Patients were divided into two groups: EARLY and LATE, by length of stay (LOS) < 9 days vs ≥ 9 days (cut off based on our institutional median LOS after PD).
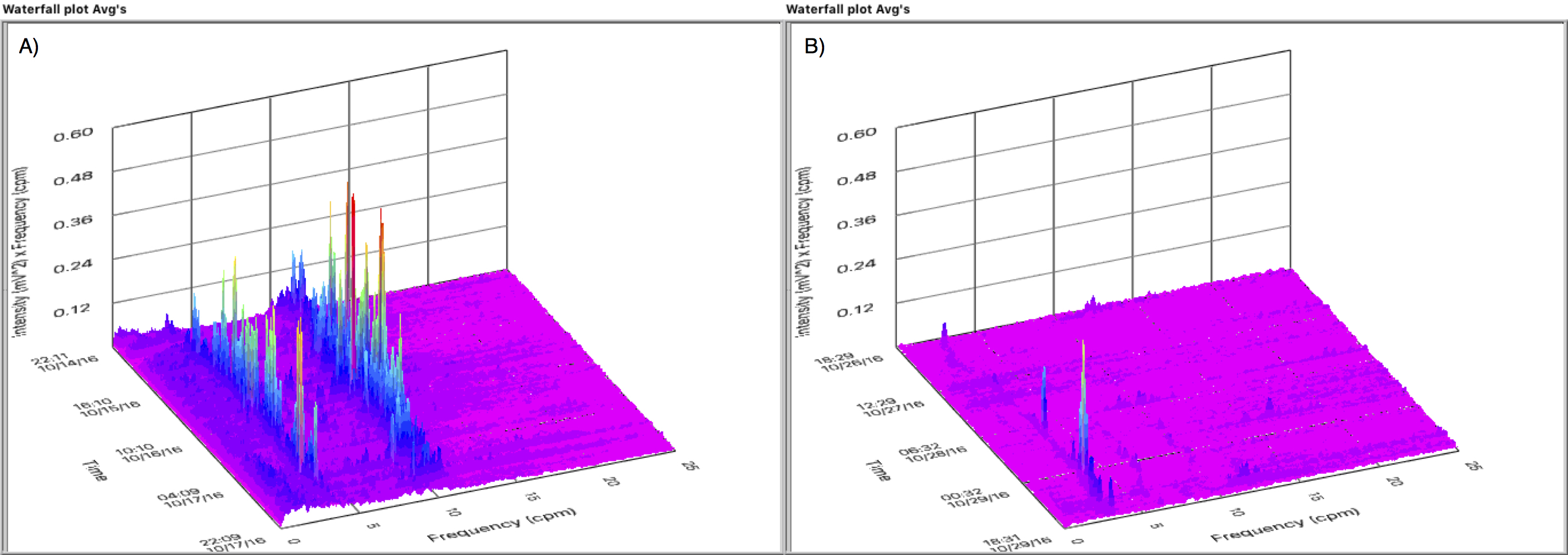
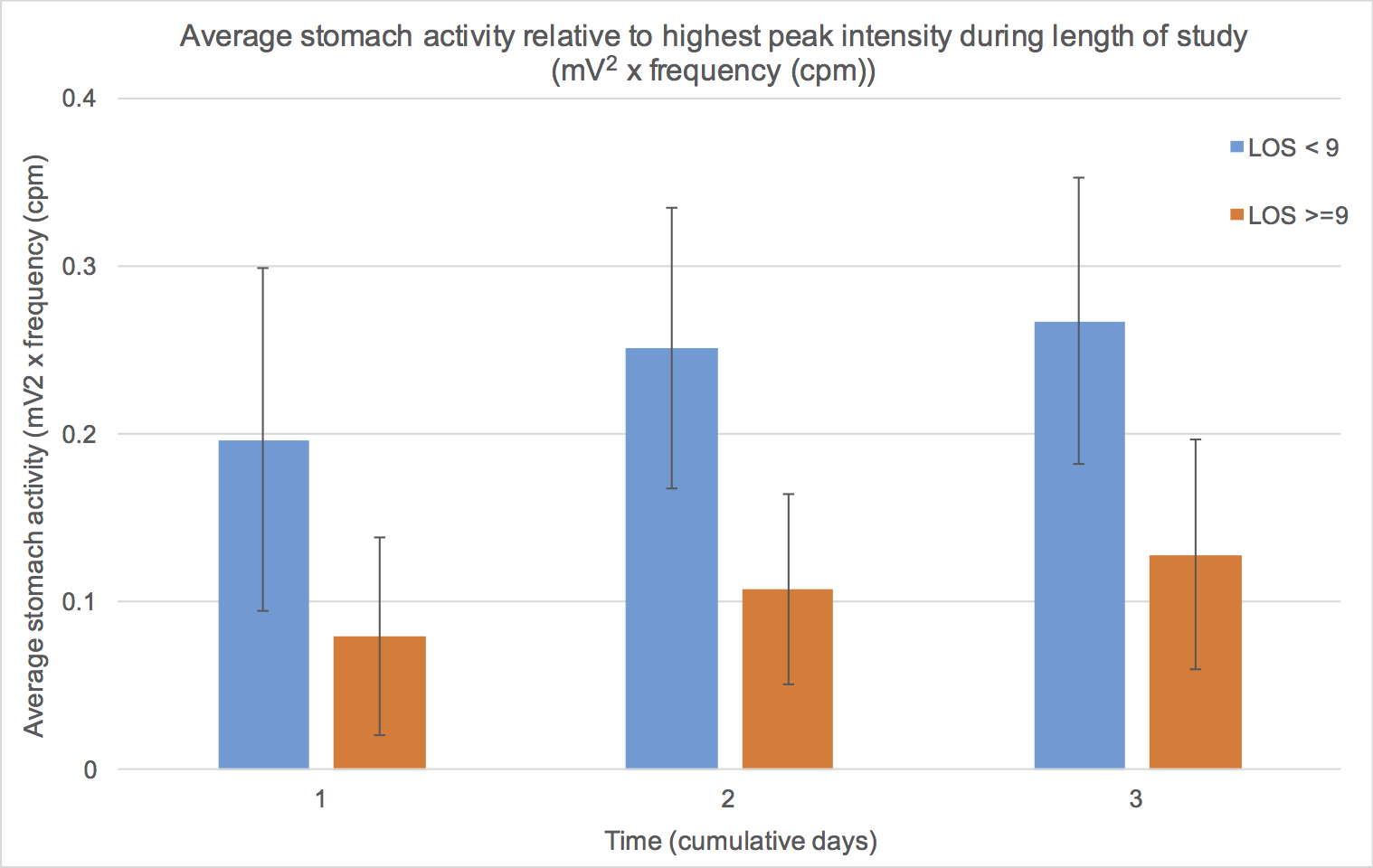
Results: Of the 30 patients enrolled, 2 were excluded for technically inadequate tracings. There were 11 patients in the EARLY and 17 in the LATE group (LOS 7 vs 11 days, respectively, p<0.05). Nasogastric insertion was required in 5 patients in the LATE group for a median of 4 days compared to no patients in the EARLY group (p=0.05). Tolerance of solid food was noted by a median of 6 days in the EARLY and 8 days in the LATE group (p<0.05). Gastric myoelectical activity was notably higher in the EARLY group versus the LATE group through the first three days after PD. Figure 1 demonstrates the waterfall plot of a representative patient in the EARLY (Fig 1A) and LATE (Fig 1B) groups demonstrating the first three days of the frequency spectrum computed every 30 minutes and staggered as a function of time. Figure 2 shows the average activity computed from days 1 through 3 for the two groups.
Conclusions: Measurement of gastric myoelectrical activity in the postoperative period after PD is feasible and can distinguish those patients with longer LOS. Further validation work is needed to develop an accurately predictive algorithm for DGE. This promising technology may allow identification of patients at risk for DGE and help guide timing of oral intake by gastric "readiness."
Back to 2017 Posters
|



