|
Back to 2017 Posters
CIRCULATING TUMOR DNA SHOULD BE CONSIDERED AS A SURROGATE MARKER FOR NEOADJUVANT CHEMOTHERAPY IN PATIENTS WITH PANCREATIC CANCER
Naoto Hadano*, Yoshiaki Murakami, Kenichiro Uemura, Naru Kondo, Naoya Nakagawa, Taijiro Sueda
Department of Surgery, Applied Life Sciences Institute of Biomedical and Health Sciences, Hiroshima, Outside U.S and Canada, Japan
Background:
Recent studies have demonstrated a clinical benefit of chemotherapy after curative surgery in patients with pancreatic ductal adenocarcinoma (PDAC), and neoadjuvant chemotherapy prior to curative surgery for patients with PDAC has been also administered to improve their prognosis. According to the National Comprehensive Cancer Network guidelines, patients who have technically resectable tumor but poor prognostic features, such as highly elevated CA19-9, large primary tumors, and large regional lymph nodes, should be considered for neoadjuvant therapy. However, there are no standard criteria for how neoadjuvant chemotherapy should be administered in patients with PDAC prior to surgery. Recently, the presence of circulating tumor DNA (ctDNA) has been reported as a useful prognostic biomarker in several cancer patients, and we have reported its utility as a prognostic biomarker in patients with PDAC. In the present study, we performed ctDNA detection in patients who were diagnosed with borderline resectable PDAC and investigated whether the presence of ctDNA can be considered as a new marker for neoadjuvant chemotherapy in patients with PDAC.
Material and method:
Blood samples were collected just prior to surgery from 50 patients who were diagnosed with borderline resectable PDAC, who then underwent pancreatic resection for PDAC at a single institution. Using droplet digital polymerase chain reaction, which allows highly precise nucleic acid quantification, we detected rare mutant tumor-derived KRAS genes from ctDNA obtained from plasma samples. Survival analyses were conducted for independent clinicopathological variables, including the presence of ctDNA.
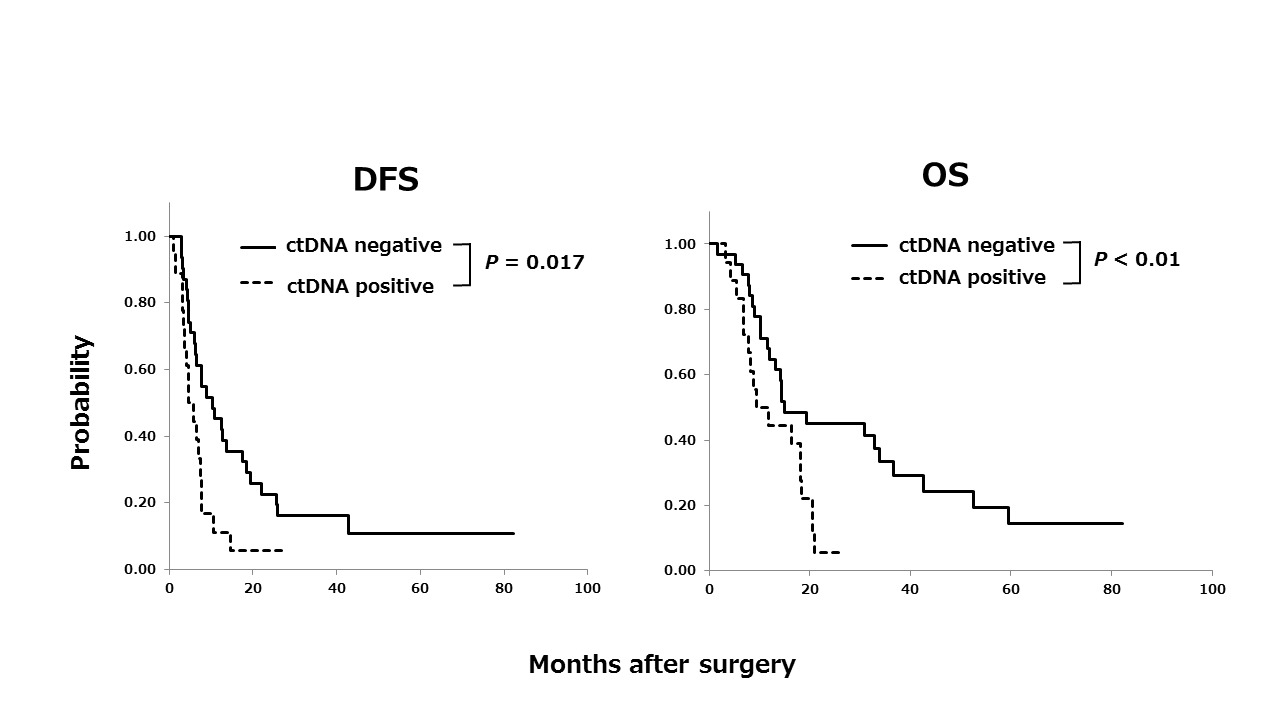
Results:
Among the 50 patients, ctDNA was detected in the plasma samples of 18 (36%) patients. The median DFS durations were 5.2 months for patients with ctDNA (ctDNA+) and 10.3 months for patients without ctDNA (ctDNA−), and the median OS durations were 10.6 months for ctDNA+ patients and 15.0 months for ctDNA− patients. ctDNA+ patients had a significantly poorer prognosis with respect to both of DFS and OS than ctDNA− patients (P = 0.017 and P < 0.01, respectively). This finding suggested that ctDNA positivity correlates with the dissemination of cancer cells into systemic circulation that cannot be identified in pre-operative imaging.
Conclusions:
Our findings suggested that the presence of ctDNA in plasma samples can be a useful and powerful marker for neoadjuvant chemotherapy in patients with borderline resectable PDAC. Accordingly, ctDNA detection may be a promising approach for deciding PDAC treatment prior to surgery.
Back to 2017 Posters
|


