
|
 |
Back to 2017 Program and Abstracts
Efficacy and Safety of Open-Label Maintenance Therapy with Subcutaneous Risankizumab in Patients with Moderate-to-Severe Crohn’s Disease
Brian G. Feagan1, Julian Panes2, Marc Ferrante3, Arthur Kaser4, Geert R. D'Haens5, William J. Sandborn6, Edouard Louis7, Markus Neurath8, Denis Franchimont9, Olivier Dewit10, Ursula Seidler11, Kyung-Jo Kim12, Christian Selinger13, Steven J. Padula14, Ivona Herichova15, Anne M. Robinson16, Kori Wallace16, Jun Zhao16, Adina I. Soaita17, Sudha Visvanathan17, David Hall17, Wulf O. Böcher14
1Robarts Clinical Trials, London, ON, Canada; 2Hospital Clinic Barcelona, Barcelona, Spain; 3University Hospitals Leuven, Leuven, Belgium; 4University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom; 5Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands; 6IBD Center University of California San Diego and UC San Diego Health System, San Diego, CA; 7University Hospital CHU Liège, Liège, Belgium; 8University of Erlangen, Erlangen, Germany; 9Erasme University Hospital, Brussels, Belgium; 10Cliniques Universitaires Saint-Luc, Brussels, Belgium; 11Hannover Medical School, Hannover, Germany; 12Asan Medical Center, Seoul, Korea (the Republic of); 13St James University Hospital, Leeds, United Kingdom; 14Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany; 15Boehringer Ingelheim RCV GmbH & Co KG, Vienna, Austria; 16AbbVie Inc., North Chicago, IL; 17Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT.
BACKGROUND: This Phase II study with risankizumab (BI 655066; ABBV-066) in patients with moderate-to-severe Crohn’s disease (CD) comprised three treatment periods: a 12-week double-blind intravenous (iv) induction period (Period 1), a 14- week open-label iv re-induction/wash-out period (Period 2) and a 26-week open-label subcutaneous (sc) maintenance period (Period 3). Risankizumab was more effective than placebo for inducing clinical and endoscopic remission at 12 weeks. Re-induction therapy with 600 mg risankizumab increased clinical remission rates further at Week 26, and was well tolerated over 26 weeks. Here, the efficacy and safety of open-label sc risankizumab maintenance therapy was assessed at Week 52.
METHODS: Patients in clinical remission (CD Activity Index [CDAI] <150) at Week 26 entered the open-label maintenance period and received 180 mg sc risankizumab every 8 weeks until study end (Week 52). At Week 26, patients with clinical response (CR-100), but not clinical remission, were offered treatment in a separate open-label long-term extension study (not included in this analysis) while patients with non-response were discontinued. Clinical response/remission and endoscopic response (>50% CD Endoscopic Index of Severity [CDEIS] reduction from baseline), endoscopic remission (CDEIS ≤4; CDEIS ≤2 for patients with isolated ileitis), and deep remission (endoscopic remission plus clinical remission) were assessed at Week 52. Non-responder imputation (NRI) was used for missing data.
RESULTS: At Week 26, 62/121 patients were in clinical remission and entered maintenance treatment, with 54 patients completing the treatment period to Week 52. At Week 52, 71.0% of patients (44/62) had clinical remission (Table 1) and 80.6% (50/62) had clinical response. Of those patients who entered Period 3 and had clinical remission at Week 12, 87.5% (21/24) were still in clinical remission at Week 52 (Table 1). The rate of endoscopic response and remission at Week 52 was 54.8% (34/62) and 35.5% (22/62), respectively. Of those patients who entered Period 3 and had endoscopic remission at Week 12, 69.2% (9/13) were still in endoscopic remission at Week 52. Overall, 29.0% of
patients (18/62) were in deep remission at Week 52. During Period 3, two patients discontinued due to an AE (worsening and exacerbation of CD; Table 2).
CONCLUSIONS: Open-label sc risankizumab was effective in maintaining clinical and endoscopic remission and response up to Week 52 in patients in clinical remission at Week 26. Early achievement of clinical or endoscopic remission at Week 12 was associated with better outcome at Week 52. Overall, risankizumab was well tolerated with no new safety signals detected during sc maintenance treatment. (Funded by Boehringer Ingelheim; ClinicalTrials.gov identifier, NCT02031276).
Table 1. Clinical remission at Week 52 by outcome of Period 1 (Week 12) and Period 2 (Week 26) for patients who entered Period 3
Table 2. Safety summary for patients who entered Period 3
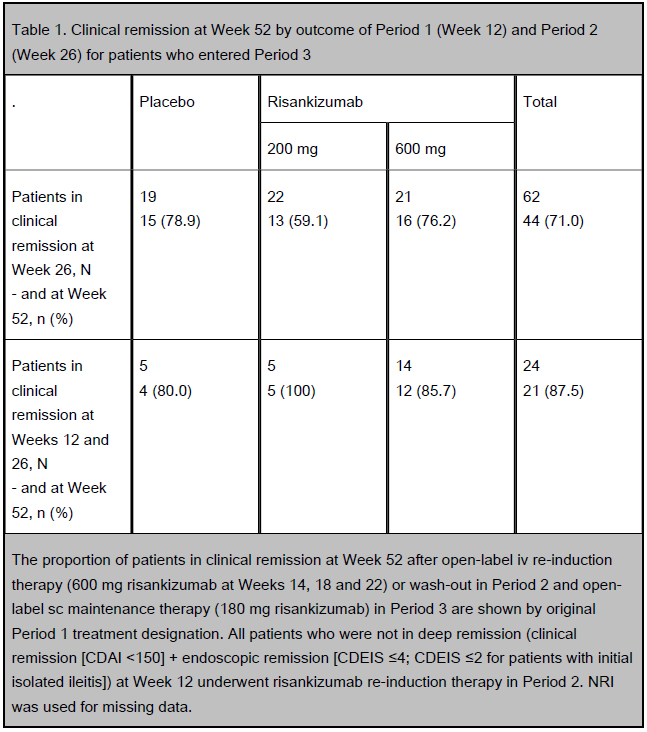
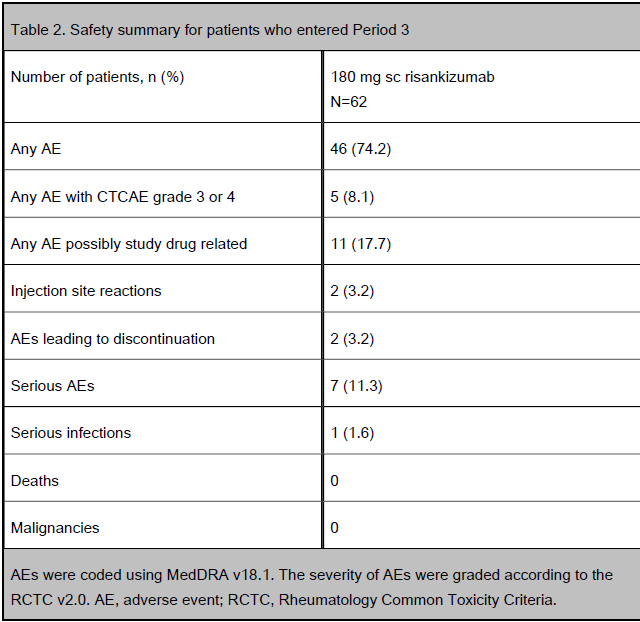
The proportion of patients in clinical remission at Week 52 after open-label iv re-induction therapy (600 mg risankizumab at Weeks 14, 18 and 22) or wash-out in Period 2 and open-label sc maintenance therapy (180 mg risankizumab) in Period 3 are shown by original Period 1 treatment designation. All patients who were not in deep remission (clinical remission [CDAI <150] + endoscopic remission [CDEIS ≤4; CDEIS ≤2 for patients with initial isolated ileitis]) at Week 12 underwent risankizumab re-induction therapy in Period 2. NRI was used for missing data. AEs were coded using MedDRA v18.1. The severity of AEs were graded according to the RCTC v2.0. AE, adverse event; RCTC, Rheumatology Common Toxicity Criteria.
Back to 2017 Program and Abstracts
|



