
|
 |
Back to 2017 Program and Abstracts
Safety and Efficacy of ABT-494 (Upadacitinib), an Oral JAK1 Inhibitor, as Induction Therapy in Patients with Crohn’s Disease: Results from Celest
William J. Sandborn1, Brian G. Feagan2, Julian Panes3, Geert R. D'Haens4, Jean Frederic Colombel5, Qian Zhou6, Bidan Huang6, Jeffrey V. Enejosa6, Aileen L. Pangan6, Ana P. Lacerda6
1University of California San Diego , La Jolla, CA; 2Robarts Clinical Trials, Western University, London, ON, Canada; 3Department of Gastroenterology, Hospital Clínic Barcelona, Barcelona, Spain; 4Academic Medical Centre, Amsterdam, Netherlands; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6AbbVie, Inc, North Chicago, IL.
Background: The efficacy and safety of ABT-494, an oral JAK1 inhibitor, were assessed in patients with moderate-to-severe Crohn’s disease (CD) who had inadequate response/intolerance to a tumor necrosis factor (TNF) antagonist.
Methods: Adults patients with active CD, with a CDAI 220-450, an average daily liquid/soft stool frequency (SF) ≥2.5 or daily abdominal pain (AP) score ≥2.0, and Simplified Endoscopic Score for CD (SES-CD) ≥6 (or ≥4 for those with isolated ileal disease), were randomized 1:1:1:1:1:1 to double-blind induction therapy with placebo (PBO) or ABT-494 at 3, 6, 12, 24 mg twice daily (BID) or 24 mg once daily (QD) for 16 weeks, followed by blinded extension therapy for 36 weeks. Patients were also randomized 1:1 at baseline for followup ileocolonoscopy at either Week 12/16. From Week 2, steroid doses were to be tapered in patients on corticosteroids at baseline. Co-primary endpoints were clinical remission (SF ≤1.5 and AP ≤1, and both not worse than baseline) at Week 16, and endoscopic remission (SES-CD ≤4 and ≥2 point reduction from baseline, no subscore >1) at Week 12/16. Secondary endpoints included clinical response (≥30% reduction from baseline in AP or SF with neither worse than baseline), and endoscopic response (≥25% decrease in SES-CD). The overall dose-response relationship between multiple ABT-494 doses and PBO was tested by Multiple Comparison Procedures Modeling (MCP-Mod); pairwise comparison between each ABT- 494 dose with PBO was tested by Cochran-Mantel-Haenszel test stratified by baseline SES-CD.
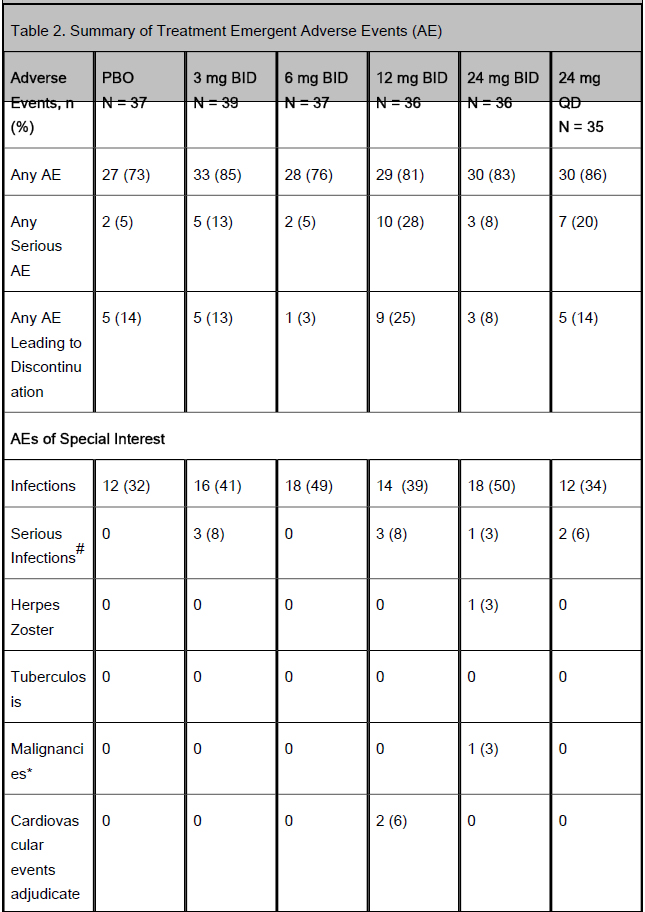
Results: Of the 220 enrolled patients, 180 (82%) completed 16 weeks of induction. Mean age was 40.7±12.9 yrs, CDAI 302.7±63.4 and disease duration 13.2±10.0 yrs. Ninety six percent had failed, or were intolerant to TNF antagonists. Significantly more patients on 6 mg BID vs PBO achieved clinical remission. A significant dose-response relationship was observed with ABT-494 vs PBO for endoscopic remission (Table 1). Compared with PBO, more patients achieved clinical response with 6 and 24 mg BID, and endoscopic response with ABT-494 doses ≥6 mg BID at Week 16 (Table 1). Adverse events (AEs) and infections were numerically higher with ABT-494 (Table 2). Serious AEs and discontinuations were similar in all groups, except for 12 mg BID. One case of non-melanoma skin cancer was reported in the 24 mg BID group. One death was reported during screening; the patient did not receive study drug. Laboratory abnormalities were mostly ≤Grade 2, with 3 events of Grade 3 hemoglobin decrease (0 on PBO) and 5 Grade 3 CPK elevations (1 on PBO/ 4 on ABT-494).
Conclusions: This dose-ranging study demonstrated endoscopic improvement and clinical benefit of ABT-494 as induction therapy in patients with moderate-to-severe refractory CD, and a safety profile as expected with a JAK inhibitor in this population.
Table 1. Efficacy Endpoints at Week 16 #µ
Table 2. Summary of Treatment Emergent Adverse Events (AE)
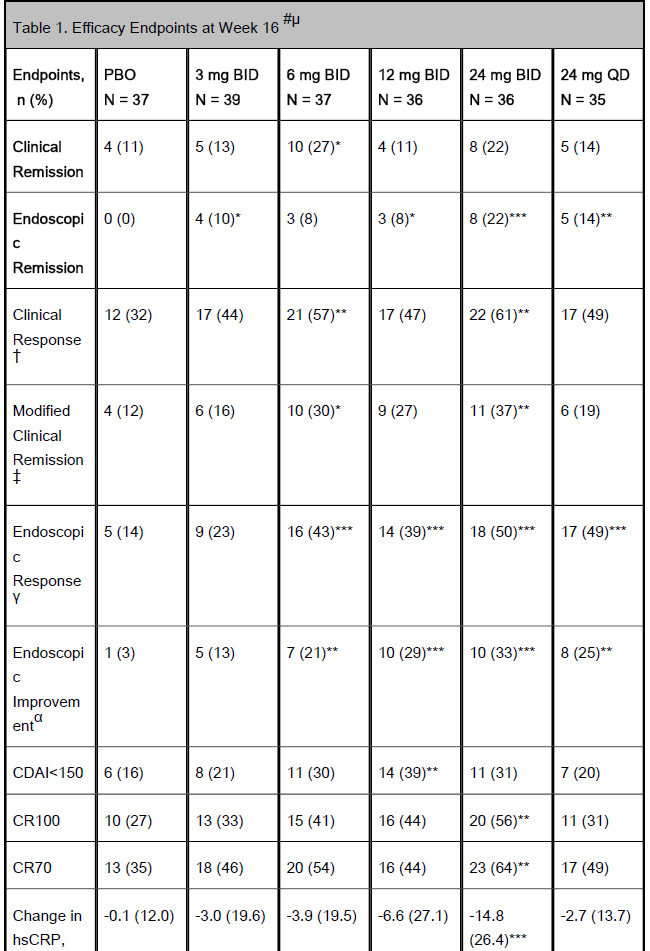
CDAI<150, Crohn’s disease activity index <150; CR70 or 100, reduction from baseline in CDAI score of 70 or 100 points.
Co-primary endpoints are in bold text.
µFor endoscopic remission, response and improvement, measurements were at Week 12/16, as scored by the central reader.
†Clinical response: ≥30% reduction from baseline in AP or SF, with neither worse than baseline.
‡Modified Clinical remission: average daily SF ≤ 2.8 and average daily AP ≤ 1.0 with both not worse than baseline.
γEndoscopic response: ≥25% decrease in SES-CD from baseline.
αEndoscopic improvement: >50% decrease in SES-CD from baseline or endoscopic remission.
# Non-responder imputation.
*, ** and ***: statistically significant at .1, .05, and .01 levels, respectively.
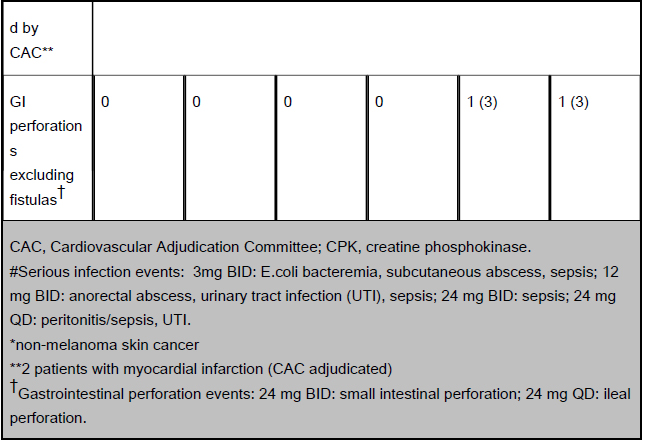
CAC, Cardiovascular Adjudication Committee; CPK, creatine phosphokinase.
#Serious infection events: 3mg BID: E.coli bacteremia, subcutaneous abscess, sepsis; 12 mg BID: anorectal abscess, urinary tract infection (UTI), sepsis; 24 mg BID: sepsis; 24 mg QD: peritonitis/sepsis, UTI.
*non-melanoma skin cancer
**2 patients with myocardial infarction (CAC adjudicated)
†Gastrointestinal perforation events: 24 mg BID: small intestinal perforation; 24 mg QD: ileal perforation.
Back to 2017 Program and Abstracts
|





