|
Back to 2017 Program and Abstracts
ENHANCING IMMUNOTHERAPY BY EPIGENETIC SILENCING OF HISTONE AND DNA METHYLATION IN PANCREATIC CANCER
Young K. Hong*, Harshul Pandit, Neil Bhutiani, Suping Li, Yan Li, Robert C. Martin
Surgery, University of Louisville, Louisville, KY
Background:Polycomb Repressor Complex 2(PRC2), a mediator of stem cell pluripotency, contributes to initiation and progression of pancreatic cancer by epigenetic silencing of tumor suppressor genes and inhibits immunomodulation of T cell migration into tumor. Enhancer of Zeste Homolog 2 (EZH2), the catalytic component of the PRC2, in addition to DNA methyltransferase (DNMT) can be targeted to reverse the transcriptionally repressive hypermethylation at the chromatin and DNA promoter level. Therefore, we aim to demonstrate that clinically available epigenetic therapy can be used on pancreatic cancer to inhibit cell growth, upregulate tumor suppressors, and induce helper T cell (Th1) chemokine expression as a feasible and therapeutic adjunct to augment immunotherapy.
Methods: Multiple Panc1 and S2-013 pancreatic adenocarcinoma cell lines were treated with 3-Deazaneplanocin A (DZNep) 5uM (EZH2 inhibitor), and 5-Azacytidine (5-AZA) 5uM (DNA methyltransferase inhibitor) in various time trials (24-48HR) followed by IFN-y 10ng/ml exposure. Cell count assay, quantitative RT-PCR (qRT-PCR), immunohistochemistry, and immunoblot were performed to evaluate the drug effect on cell growth, tumor suppressor, and chemokine gene expression after treatment with DZNep, 5-AZA, IFN-y, and combination drug therapy.
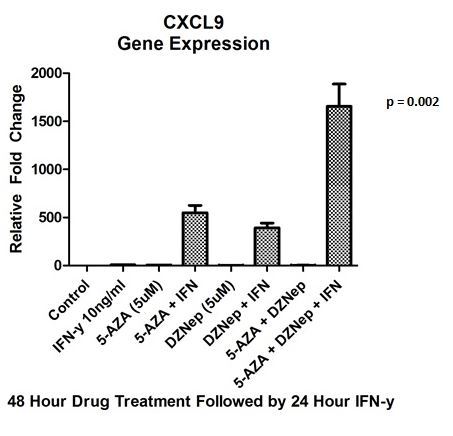
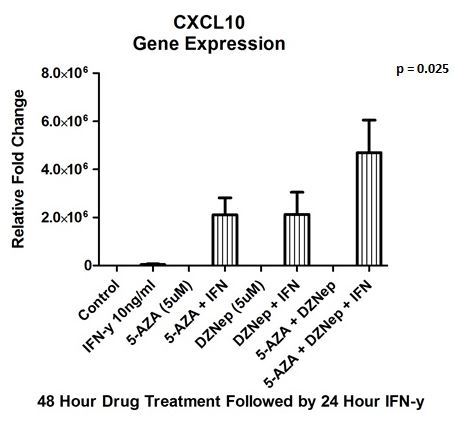
Results: Pancreatic cancer cell lines demonstrated effective inhibition of cell growth with DZNep and 5-AZA in a dose- and time dependent manner compared to untreated control (68% and 79%, respectively; p = 0.009). The qRT-PCR assay revealed a significant upregulation of immunomodulating T helper (Th1) chemokines, CXCL9 (1.65 x 103 +/- 231 rel. fold change; p = 0.002) and CXCL10 (4.69 x 106 +/- 1.3x106 rel. fold change; p = 0.025) after dual epigenetic drug treatment and stimulation with IFN-y (Figure 1&2). Furthermore, there was an upregulation of expression of previously transcriptionally silent tumor suppressor genes p21(29.9 +/- 0.42; p = 2.0 x 10-7), p53 (3.8 +/- 0.47; p = 0.004), and p57 (4.1 +/- 1.42; p = 0.09).
Conclusion: Epigenetic therapy can be utilized in pancreatic cancer by reversing the gene inhibitory methylation marks and upregulating repressed tumor suppressor. There is a potential role of augmenting immunotherapy at the time of diagnosis in pancreatic cancer by upregulation of immunomodulating chemokines CXCL9 and CXCL10, which would enhance local and systemic response rates through migration of T lymphocyte into the tumor.
Back to 2017 Program and Abstracts
|

