|
Back to 2017 Program and Abstracts
PORTAL VEIN EMBOLIZATION REDUCES POSTOPERATIVE HEPATIC INSUFFICIENCY ASSOCIATED WITH HEPATIC ATROPHY CAUSED BY EXTENSIVE CHEMOTHERAPY
Suguru Yamashita*1,2, Kiyohiko Omichi1, Claudius Conrad1, Yun Shin Chun1, Thomas Aloia1, Jean Nicolas Vauthey1, Ching-Wei Tzeng1
1Surgical oncology, University of Texas MD Anderson Cancer Center, Tokyo, Japan; 2Hepato-Biliary-Pancreatic Surgery Division, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine, the University of Tokyo, Tokyo, Japan
Background:
Extensive preoperative chemotherapy is associated with both increased postoperative hepatic insufficiency (PHI) and death from liver failure after major hepatectomy. Using portal vein embolization (PVE), the generation of adequate degree of hypertrophy (5%) and kinetic growth rate (KGR, 2%/week) of the future liver remnant (FLR) accurately predicts and reduces rates of PHI. Further studies have recently suggested that a higher degree of atrophy of the total liver volume (TLV) during preoperative chemotherapy is correlated with increased rates of PHI. The primary aim of this study was to evaluate whether PVE could protect against PHI from major hepatectomy in patients with a higher degree of atrophy after extensive preoperative chemotherapy.
Methods:
Clinicopathological features of patients who received preoperative chemotherapy and underwent hepatic resection after PVE were reviewed from a single institution (1995-2015). Degree of atrophy was defined as the percentage difference of TLV (estimated by manual volumetry) divided by the standardized total liver volume (sTLV) ratio, or ([pre-chemotherapy TLV] - [post-chemotherapy TLV]) × 100 ÷ sTLV (%). KGR was defined as the degree of hypertrophy (absolute percentage change in FLR volume from baseline) divided by the number of weeks after PVE. KGR and event rates of PHI and death from liver failure were compared between patients with degree of atrophy <10% vs. ≥10%. A multivariate model was used to identify the independent predictors of PHI.
Results:
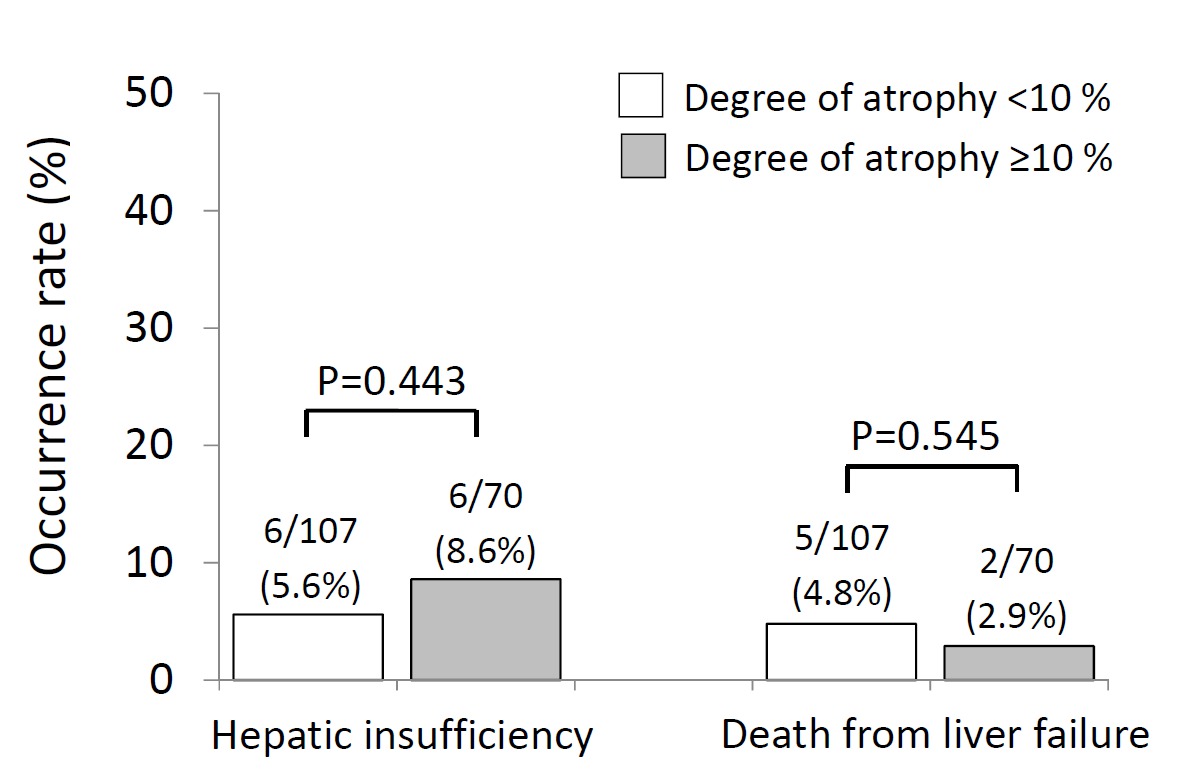
Among 278 total patients undergoing PVE/resection, 177 patients (90% colorectal liver metastases) received preoperative chemotherapy and were included in the study. Rates of PHI (5.6% vs. 8.6%, p=0.443) and death from liver failure (4.8% vs. 2.9%, p=0.545) were similar between patients with degree of atrophy <10% vs. ≥10% (Figure 1). KGR <2 %/week (odds ratio [OR] 9.03, p=0.009) was the sole independent preoperative predictor of PHI. Grade B/C bile leak (OR 6.56, p=0.021) was the only postoperative predictor of PHI.
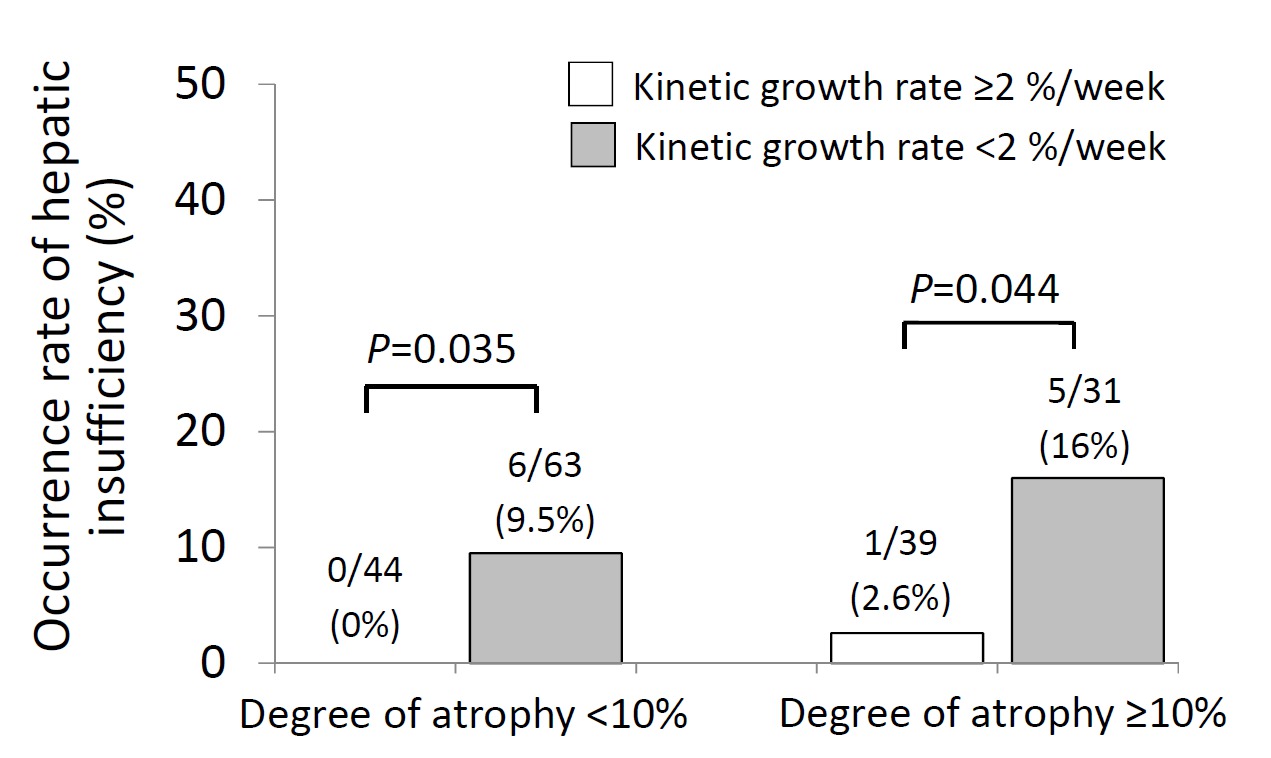
Patients with both degree of atrophy ≥10% and KGR <2% had a 16% (5/31) rate of PHI vs. 0% (0/44) in those with both degree of atrophy <10% and KGR ≥2% (p=0.006). In both cohorts of patients, adequate KGR ≥2% reduced PHI (from 9.5% to 0% in the atrophy<10% patients, p=0.035, and from 16% to 2.6% in the atrophy≥10% patients, p=0.044, Figure 2).
Conclusions:
Even in high-risk patients with a degree of atrophy ≥10% from preoperative chemotherapy, KGR ≥2% mitigates the deleterious effects of hepatic atrophy and significantly reduces PHI to almost zero. In patients at risk for PHI due to hepatic atrophy, PVE with KGR calculation remains the most important preoperative technique to reduce liver failure after major hepatectomy.
Back to 2017 Program and Abstracts
|



