|
Back to 2017 Program and Abstracts
CANCER ASSOCIATED FIBROBLASTS MEDIATE RESISTANCE TO RADIOTHERAPY IN RECTAL CANCER CELLS
David Liska*, Shao Xiang, Georgios Karagkounis, Steven Signs, Matthew Kalady, Emina Huang
Department of Colorectal Surgery, Cleveland Clinic, Cleveland, OH
Introduction: The standard of care for locally advanced rectal cancer includes neoadjuvant chemoradiotherapy followed by surgical resection. The tumor response to chemoradiotherapy has been shown to be an independent predictor of oncologic outcomes. However, the response to treatment is highly variable. The biological reason for this variable response to radiotherapy is unknown. Chemoradiotherapy leads to an expansion of cancer associated fibroblasts (CAFs) in the rectal tumor microenvironment, thus signifying their potential role in the response to treatment. We hypothesize that CAFs mediate resistance to radiation by secreting cytokines that act on cancer cells in a paracrine fashion.
Methods: Primary CAFs were isolated and propagated from rectal cancer patients who had not received neoadjuvant therapy. Immunofluorescence was used to confirm the expression of CAF specific markers. CAFs were treated with 10Gy of ionizing radiation and conditioned media (CM) was harvested. CAFs effect on in vitro migration of rectal cancer cells (SW837, HRT-18; ATCC) was assayed by means of wound healing assays using a standardized culture insert. Rectal cancer cells were co-injected with radiated or non-radiated CAFs subcutaneously into the flanks of NSG mice and tumor growth was assessed. Cytokine array analysis was used to determine the effect of radiation on the CAF secretome.
Results: CAFs isolated from fresh rectal cancer specimens stained positively for the activated fibroblast markers vimentin and alpha-SMA, and negatively for the epithelial marker cytokeratin 19, confirming CAF lineage. Activation of CAFs by radiation releases factors that significantly augmented the migration of rectal cancer cells in vitro in wound healing assays (Figure 1). Radiated CAFs co-injected with rectal cancer cells as flank xenografts augmented in vivo tumor growth when compared to control and non-radiated CAFs (Figure 2). Cytokine array analysis of two different rectal CAF cell lines (CA115FB, CA36FB) showed that radiation increased the secretion of the factors IL8 (CA115FB and CA36FB), IL6 (CA36FB), bFGF (CA36FB), and GRO (CA115FB and CA36GF). These could represent potential targets to disrupt the pro-tumorigenic contributions of the tumor microenvironment during neoadjuvant treatment.
Conclusion: CAFs present in the rectal tumor microenvironment, when treated with radiation, increase the secretion of factors that act on rectal cancer cells in a paracrine fashion contributing to tumor growth and cell migration. Targeting the paracrine contribution of CAFs in the tumor microenvironment is a potential strategy to augment the effectiveness of neoadjuvant therapy.
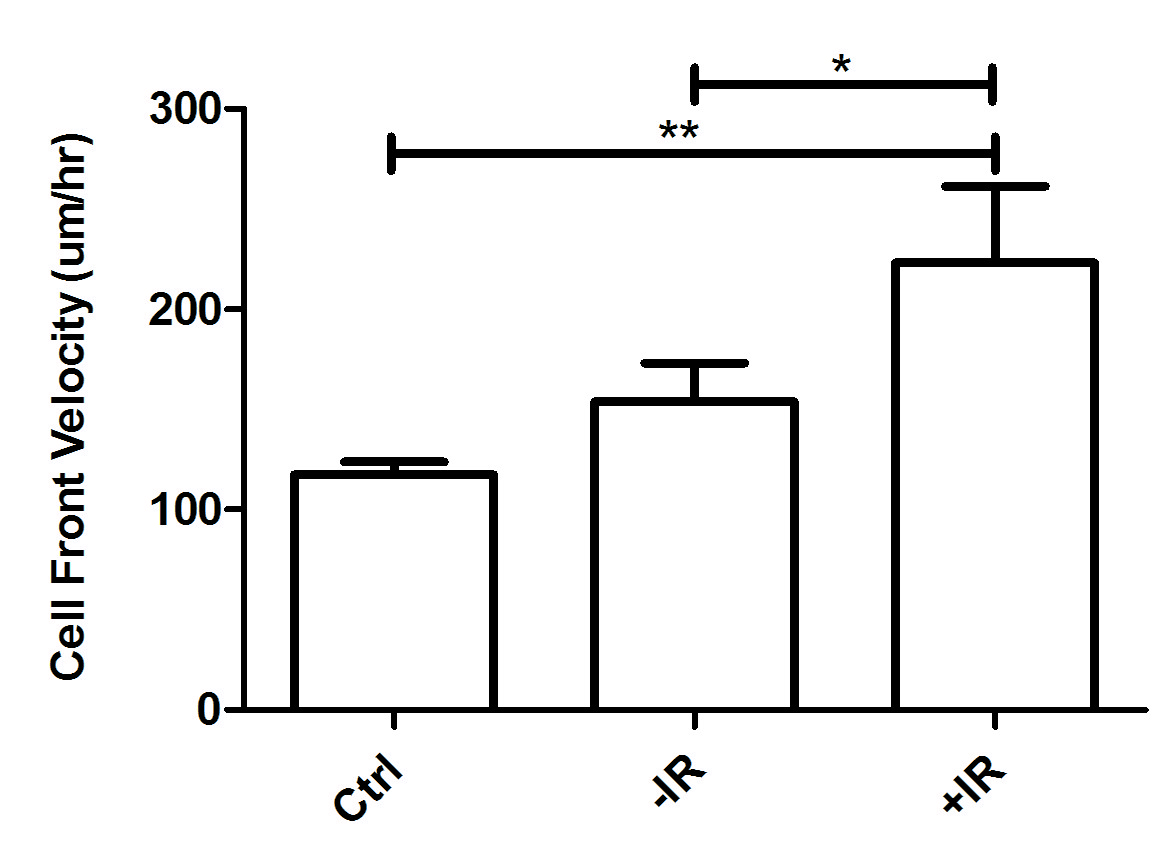
Figure 1: Wound healing assay of rectal cancer cells (SW837) treated with conditioned media from non-radiated (-IR), and radiated (+IR) rectal CAFs (CA115FB).
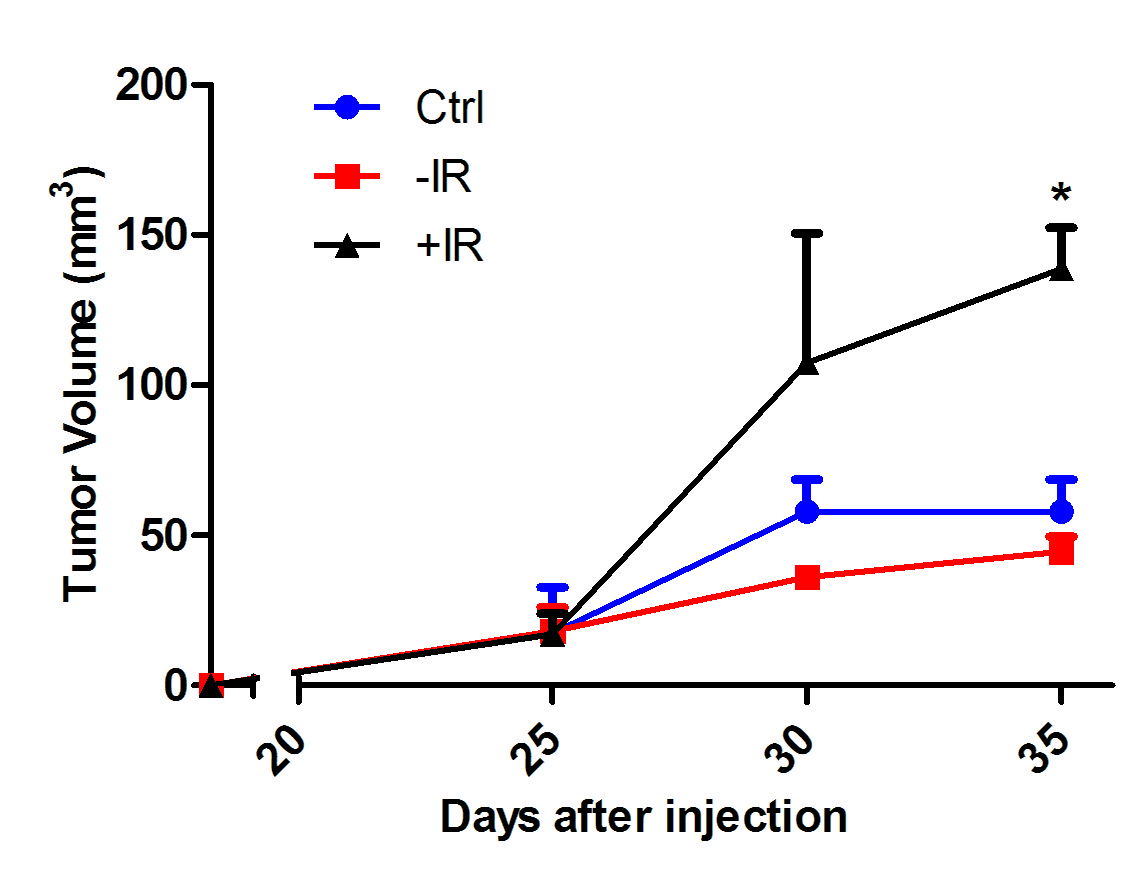
Figure 2: Tumor growth of rectal cancer cell (HRT-18) xenografts, co-injected with non-radiated (-IR) and radiated (+IR) rectal CAFs (CA115FB).
Back to 2017 Program and Abstracts
|



