
|
 |
Back to 2017 Program and Abstracts
BETTER LATE THAN NEVER?: AN EXAMINATION OF ADHERENCE TO ADJUVANT THERAPY GUIDELINES FOR STAGE III COLON CANCER IN AN UNDERSERVED REGION
Whitney Guerrero*1, Amy Wise2, Garrett Lim2, Lei Dong1, Jim Wan1, Jeremiah Deneve1, Evan Glazer1, Paxton Dickson1, Ray S. Daugherty1, Martin Fleming1, David Shibata1
1Surgery, University of Tennessee Health Science Center Memphis, Memphis, TN; 2College of Medicine, University of Tennessee Health Science Center, Memphis, Memphis, TN
Introduction
In 2008, the American College of Surgeons Commission on Cancer (CoC) issued guidelines to standardize quality of care (QOC) for Stage III colon cancer. The recommendation for node-positive disease is initiation of adjuvant chemotherapy (AC) within 120 days of diagnosis. We sought to examine the rates and impact of adherence and non-adherence in a healthcare system serving a region identified as having significant disparities in colon cancer outcomes.
Method
An IRB-approved retrospective analysis was performed of patients treated for Stage III colon cancer in a multi-hospital healthcare system within an underserved region from 2005-2014. The association between adherence and clinicopathologic, demographic, geographic and socioeconomic data were examined. Overall survival rates were compared between guideline compliant (GC) and non-compliant (nGC) groups.
Results
Of 1171 colon cancer patients, 524 had Stage III disease with 64% (n=333) and 36% (n=191) receiving and not receiving AC respectively. As expected, AC conferred a significant survival advantage with a median survival of 77.7 months (IQR 38.3-96.7) among patients receiving AC compared to 40.5 months (IQR 11.1-58.7) for patients who did not (p<0.0001). Patients who received AC were significantly younger (60 +/- 12 years vs. 67 +/- 15 years, p<0.0001) and less likely to be African-American than those who did not (44% v 55%, p=0.01). Of those who received AC, 73% (n=244) started treatment within 120 days (GC) whereas 37% (n=89) started treatment beyond 120 days (nGC). We found no statistically significant demographic, geographic or socioeconomic differences between the GC and nGC groups. The nGC group did have significantly longer follow up (50.5 months v. 37.5 months, p<0.0001). There was no statistically significant survival difference observed between the GC and nGC groups (p=0.1, Figure 1A). We observed statistically significant differences when patients who received no chemotherapy (NC) were compared to GC and nGC groups (p<0.0001 for both comparisons, Figures 1B).
Conclusion
Rates of compliance with CoC guidelines for timing of adjuvant therapy for Stage III colon cancer in our system are lower than the CoC Estimated Performance Rate (EPR) for 2014 (64% v. EPR 72.4%). In our population, age and race influenced the likelihood of receiving AC. Notably, the administration of AC, even when administered beyond the recommended time frame, conferred a significant survival benefit. This quality measure represents an important target for improving colon cancer outcomes in this region.
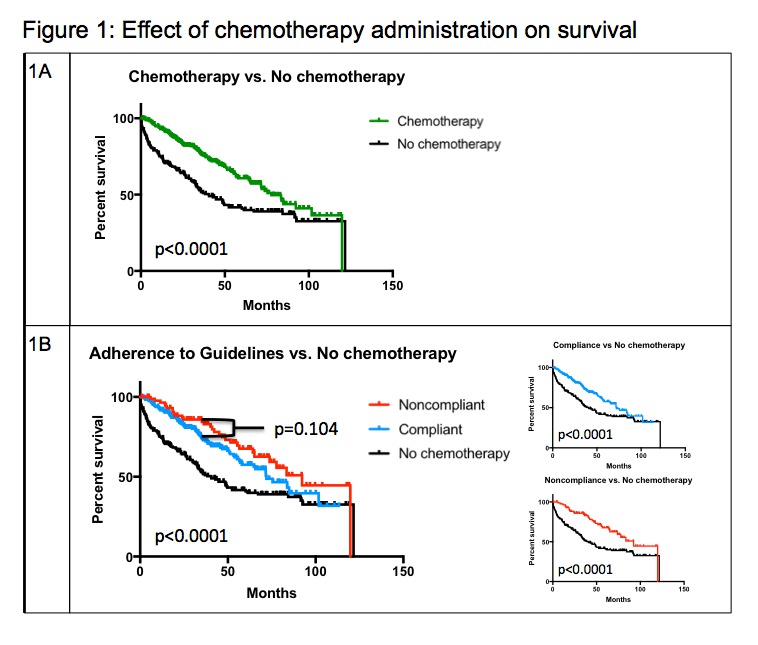
1A: Adjuvant chemotherapy confers a significant survival benefit over treatment without chemotherapy
1B: While the difference between GC and nGC survival is not statistically significant, both GC and nGC groups saw survival advantages over the NC group
Back to 2017 Program and Abstracts
|


