|
Back to 2015 Annual Meeting Program
Hepatic Application of Ablative Thermal Energy Promotes Pro-Tumorigenic Alternative Activation of Peritoneal Macrophages via Hypoxia Inducible Factor-1α
Jeniann Yi*1, Karim C. El Kasmi3, Brooke C. Bredbeck1, Gregory Jurkovich2, Richard D. Schulick1, Barish H. Edil1, Carlton Barnett1, 2
1Department of Surgery, University of Colorado, Aurora, CO; 2Department of Surgery, Denver Health Medical Center, Denver, CO; 3Department of Gastroenterology, University of Colorado, Aurora, CO
Introduction: We have shown that inhibition of hypoxia inducible factor-1α (HIF1α), a transcription factor associated with advanced malignancy, mitigates local tumor recurrence after hepatic application of ablative thermal energy with electrocautery and radiofrequency ablation (RFA) in a murine model of metastatic colon cancer. Macrophage activation is characterized broadly as M1/classical or M2/alternative, with the M2 phenotype being pro-tumorigenic. We hypothesize that the tumorigenic response to hepatic thermal injury is mediated by peritoneal-derived macrophages (PDMs) that are alternatively activated via HIF1α activity.
Methods: C57/Bl6 mice underwent hepatic ablative thermal energy application via a small laparotomy incision. Energy was applied at 90°C for 30 seconds to create a single lesion in the right lower lobe of the liver. Control mice underwent sham laparotomy. After 1 week, mice were sacrificed and PDMs were harvested via phosphate buffered saline irrigation. Cells were passed in culture to select for PDMs, and lysed with mRNA harvested for real-time polymerase chain reaction (RT-PCR) normalized to sham PDMs to detect target genes of macrophage activation pathways. Statistical analysis was performed using GraphPad Prism with α<0.05.
Results: Following hepatic ablative thermal energy application, PDMs showed significantly decreased expression of the M1 marker SOCS1 (p<0.0001) and increased expression of the M2 markers SOCS3 (p=0.0034) and arginase-1 (p<0.0001). Testing of the canonical M2 pathway marker IL-4 receptor-α similarly had increased expression after thermal energy application (p<0.0001). Testing for cytokines IL-6 and IL-1β also demonstrated increased expression as compared to sham PDMs, representing an additional pathway of M2 activation amongst PDMs following thermal energy application. Regarding HIF1α, PDMs after hepatic thermal energy application showed a significant increase in HIF1α expression (p<0.0001) as well as a 40-fold increase in VEGF expression, a target gene of HIF1α.
Conclusion: Hepatic application of ablative thermal energy promotes alternative activation of peritoneal macrophages. Clinically, alternatively activated macrophages are associated with poor patient prognosis, as they are believed to suppress the anti-cancer immune response within the tumor microenvironment. Further, hepatic application of ablative thermal energy may promote tumor progression via increased expression of HIF1α targets such as VEGF. Modulation of these pathways may improve the therapeutic benefit of extirpative therapies.
Figure 1. Relative expression of macrophage phenotype markers SOCS1 and SOCS3 among treatment groups.
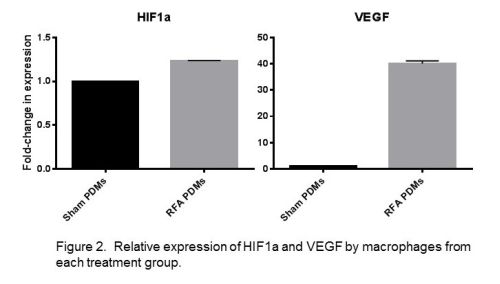 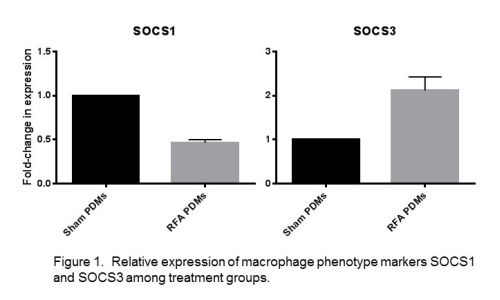
Figure 1. Relative expression of macrophage phenotype markers SOCS1 and SOCS3 among treatment groups.

Figure 2. Relative expression of HIF1α and VEGF by macrophages from each treatment group.
Back to 2015 Annual Meeting Program
|



