
|
 |
Back to 2015 Annual Meeting Program
The Impact of Surgical Excisions on Human Gastric Electrical Conduction
Ahmer Hameed*1, Peng Du2, Timothy R. Angeli2, Christopher J. Lahr3, Thomas L. Abell4, Leo K. Cheng2, 5, Gregory O'Grady2, 6
1Department of Surgery, Westmead Hospital, Eastwood, NSW, Australia; 2Auckland Bioengineering Institute, Auckland, New Zealand; 3Mississippi Medical Center, Department of Surgery, Jackson, MS; 4Department of Gastroenterology, University of Louisville, Louisville, KY; 5Department of Surgery, Vanderbilt University, Nashville, TN; 6Department of Surgery, University of Auckland, Auckland, New Zealand
Introduction:
Gastric contractions are coordinated by slow waves, generated by interstitial cells of Cajal (ICC). Gastric surgery alters slow wave conduction, possibly contributing to post-operative nausea and gastric dysfunction. However, the impact of gastric incisions and excisions on slow waves has never been assessed in accurate spatiotemporal detail. We applied high-resolution (HR) electrical mapping and in-silico modeling to define how excisions affect slow wave conduction.
Methods:
Patients with gastroparesis undergoing stimulator implantation were recruited (n=10). Full thickness stapled excisions (2x1.5 cm) were performed in the distal corpus for histological sampling, and intraoperative HR mapping (256 electrodes; 36cm2) was performed over and around excision sites. A mathematical model of three coupled biophysically-based ICC layers was developed to simulate slow wave propagation, and this was applied to comprehensively investigate the effects of different excision orientations on slow waves.
Results:
Normal gastric activation propagated aborally with wavefronts oriented transverse to the organ axis (3.0 ± 0.2 cycles/min). Excisions induced complete conduction block. Wavefronts rotated around the ends of these blocks, followed by rapid circumferential activation distal to the block (8.5 ± 1.2 mm/s vs normal 3.6 ± 0.4 mm/s; P<0.01). This conduction anisotropy rapidly restored normal antegrade propagating wavefronts distal to excisions (Figure 1). Excisions contributed to a range of complex arrhythmias observed in 4/10 patients, including retrograde activation (3 pts), ectopic pacemaking (2), functional blocks (2) and wave collisions (1). The simulations demonstrated that velocity anisotropy was explained by bidirectional coupling within ICC networks, and that anisotropy was highest when excisions were oriented in the transverse axis (0.31 ± 0.04 at 0o/180o vs 0.06 ± 0.01 at 90o).
Conclusions:
Placing incisions in the longitudinal gastric axis causes least disruption to electrical conduction. However, if transverse incisions are made, a homeostatic mechanism of conduction anisotropy compensates by rapidly restoring aborally-propagating wavefronts. These results can be explained by bidirectional coupling within ICC networks. Arrhythmias may arise after excisions, shown here in gastroparetics, possibly contributing to post-operative gastric dysfunction.
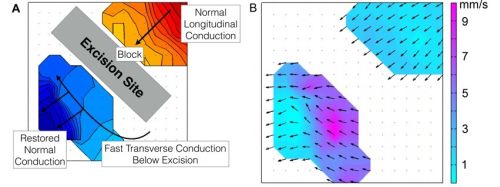
Figure 1:
Representative electrical mapping results. A) Conduction map over a gastric excision. Each color step (red to blue) represents the area of slow wave propagation per 1 s. B) Velocity map of the same wavefront in (A), demonstrating conduction anisotropy distal to the excision site.
Back to 2015 Annual Meeting Program
|


