|
Back to 2015 Annual Meeting Program
Adipose Derived Mesenchymal Stem Cells in Fistula Treatment - Prospective Study on Experimental Model of Perianal Crohn's Disease.
Ondrej Ryska*1, Zuzana Serclova1, Ondrej Mestak2, Eva Matouskova3, Pavel Vesely2, Iveta Mrazova4
1Department of Surgery, NH Hospital, Horovice, Czech Republic; 2Department of Plastic Surgery, Hospital Bulovka, Prague, Czech Republic; 3Department of Burns Medicine, 3rd Medical Faculty Charles University, Prague, Czech Republic; 4Centrum of Experimental Medicine, Institute for Clinical and Experimental Medicine, Prague, Czech Republic
Adipose derived mesenchymal stem cells in fistula treatment - prospective study on experimental model of perianal Crohn's disease.
Ryska O, Serclova Z, Mestak O, Matouskova E, Vesely P, Mrazova I
Background:
Conservative and surgical treatment of perianal fistulas in patients with Crohn's disease (CD) is still not enough effective. Surgical therapy usually requires major transanal surgery with long recovery. Local administration of adipose tissue derived stem cells (ADSCs) or biomaterials represents new approach in human fistula treatment with mixed results. In vivo bioluminescence imaging using firefly luciferase can clarify the distribution and viability of the ADSCs after implantation.
Aim:
The aim of the study was to evaluate the fistula healing after local application of ADSCs in comparison with other surgical and non-surgical techniques
Methods:
Coecostomy was used as a fistula model in Lewis rats. The inguinal adipose tissue was harvested from transgenic donor expressing firefly luciferase (LEW-Tg(Rosa-luc)11Jmsk; Jichi Medical School, Japan). Using collagenase technique suspension of vital ADCSs (1-2*106 cells/ml) was obtained.
Rats were randomized into the following interventional groups:
Group A (N=12) - fistula tract ligation
Group B (N=16) - perifistular application of ADSCs
Group C (N=12) - intrafistular application of flowable cross-linked collagen and glycosaminoglycan matrix - Integra® followed by perifistular application of ADSCs and external opening suture.
Group D (N=11)- intrafistular application of 70% glucose followed by external opening suture.
Fistula drainage assessment (FDA) was used to evaluate the fistula healing. Rats were imaged in IVIS Lumina XR camera during 30days follow-up. Interventional groups were compared with untreated (N=30) resp. external opening sutured fistulas (N=9).
The protocol was approved by Committee for the Protection of the Animals of the Czech Academy of Sciences.
Results:
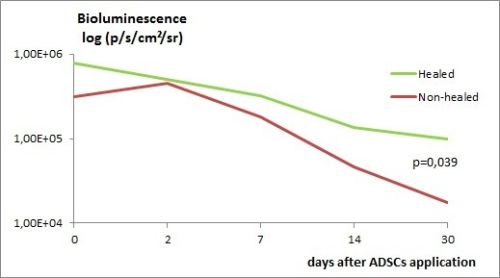
There was no mortality after interventions. Fistula was healed in 6 (50.0%) animals in group A and 6 (37.5%) in group B, which was significantly more frequent than in untreated control (spontaneous closure in 1 (3%) case - p< 0.01). In groups C and D the fistula closure occurred in 5 (41.7%) resp. 3 (27.3%) animals, which did not differ significantly from the control group - 3 (33.3%), p>0.05. In group B, the bioluminescence signal 30 days after application was higher in animals with closed fistula - 8.23 (1.20 to 16.9)*104 vs. 1.74 (0.16-6.9)*104 p/s/cm<+>2<+>/sr (p = 0.039) - graph 1.
Conclusion:
Presence of viable ADCSc after perifistular application was associated with fistula closure. Results of this technique were comparable with surgical treatment (fistula tract ligation). Application of biomaterials or 70% glucose into the fistula tract did not improve healing.
Supported by IGA13708
Back to 2015 Annual Meeting Program
|


