|
Back to 2015 Annual Meeting Program
A Novel Immunocompetent Orthotopic Mouse Model of Pancreatic Cancer With Robust Stroma: a Unique Tool for Evaluation of Therapies
Kaustav Majumder*, Shrey Modi, Nivedita Arora, Rohit Chugh, Alice Nomura, Patricia Dauer, Sulagna Banerjee, Rajinder Dawra, Ashok Saluja, Vikas Dudeja
Surgery, University of Minnesota, Minneapolis, MN
BACKGROUND: The development of novel therapeutics for pancreatic cancer has been hindered by a lack of relevant preclinical models. A valid tumor model has to recapitulate the tumorigenic properties as well as immune and stromal microenvironment which is absent in immunodeficient mice models (SCID, Athymic nude). While these components are present in the KPC (Pdx-Cre KrasG12D/+ p53−/−) model, this genetic model has an immense variability in time to invasive disease (47-355 days of life) which makes it unsuitable for the evaluation of novel therapies. In this study we have developed a novel orthotopic tumor model with tumors from KPC mice implanted in C57Black6 mice to address the shortcomings of previous models. Using this novel model we have evaluated the efficacy of Minnelide, a water soluble analog of triptolide, which was developed in our laboratory and is currently in Phase I clinical trials.
METHODS: Pancreatic tumors were extracted from 6 month KPC and cut into ~3mm3 pieces. Laparotomy was performed on C57BL/6 mice and a tumor piece was sewn into a pocket of pancreas using a figure-of-8 stitch of 7-0 prolene. After 4-8 weeks, animals were euthanized and tumors collected. Stromal components and immune cell infiltration were studied by IHC and flow cytometry. In a separate model, the effect of Minnelide (0.42mg/kg/day) on tumor growth and microenvironment was evaluated.
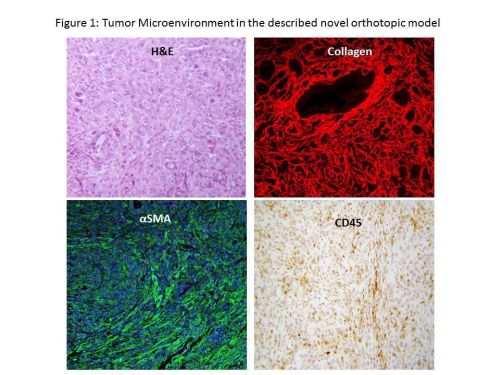
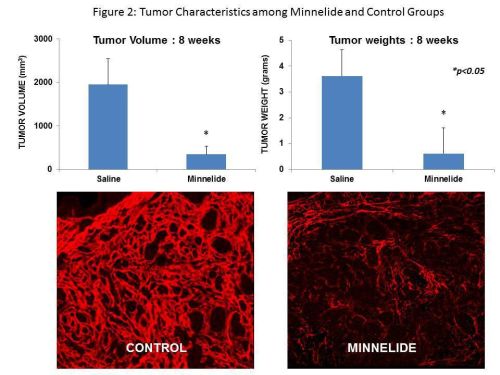
RESULTS: Tumor take rate was 90%. Consistent tumor growth was observed with time [Tumor volume (mm3, Mean ± SD); 4 wk: 1101±347, 8 wk: 1958±590]. At 8 weeks, 30% of mice had died due to tumor burden suggesting that this model can be used to evaluate the impact of novel therapies on disease specific survival. Staining for stromal components (collagen and αSMA) and immune markers (CD45) showed intense desmoplasia as well as infiltration of tumor and surrounding pancreas with leukocytes respectively (Figure1). Flow cytometric analysis of tumor cellular composition showed infiltration with macrophages, myeloid derived suppressor cells and regulatory T cells. As shown in Figure 2, Minnelide treatment resulted in a significant decrease in the tumor volumes and weight [Tumor volume (mm3, mean ± SD). 4 wk: Minnelide 62± 19 vs Control 1101± 347 mm3, 8 wk: Minnelide 368± 180 vs Control 1985 ± 590 mm3, p<0.05]. Furthermore, Minnelide treatment significantly decreased the stromal collagen content (Figure 2) and CD4 infiltrate [24± 3% vs 36±4%, Minnelide vs Control, p<0.05].
CONCLUSIONS: Our orthotopic model demonstrates consistent growth rate, tumor associated mortality and recapitulates the tumor microenvironment of human pancreatic ductal adenocarcinoma in terms of stroma components and immune infiltration. It also circumvents the variability seen in previous mouse models. This clinically relevant model can be a valuable tool to evaluate novel therapies in pancreatic cancer.
Back to 2015 Annual Meeting Program
|



