
|
 |
Back to 2014 Annual Meeting Abstracts
Does Drain Fluid Amylase Accurately Predict Pancreatic Fistula?
Christina W. Lee*1, Henry Pitt2, Taylor S. Riall3, Sean Ronnekleiv-Kelly1, Jacqueline S. Israel1, Glen Leverson1, Abhishek Parmar3, E. M. Kilbane4, Bruce L. Hall5, Sharon M. Weber1
1Surgery, University of Wisconsin Hospital and Clinics, Madison, WI; 2Surgery, Temple University Health System, Philadelphia, PA; 3Surgery, University of Texas Medical Branch, Galveston, TX; 4Surgery, Indiana University Health, Indianapolis, IN; 5Surgery, Washington University, St. Louis, MO
BACKGROUND/INTRODUCTION:
Improvements in the ability to predict pancreatic fistula (PF) could enhance patient outcome by early drain removal and avoidance of drain-related morbidity in those who will not develop PF. Although clinical predictors are associated with an increased risk for PF, these are imprecise. Previous studies have shown that drain fluid amylase on post operative day 1 (DFA1) >5000 is predictive of PF. Therefore, we sought to assess the accuracy of DFA1 to predict PF.
METHODS:
Patients undergoing pancreatic resection from 11/1/11 to 12/31/12 were selected from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Pancreatectomy Demonstration Project (PDP) database. PF was defined as persistent drainage of amylase-rich fluid in addition to drain continuation > 7 days, percutaneous drainage of pancreatic fluid collection, or re-operation. Factors significantly associated with pancreatic fistula on univariate analysis (p < 0.1) were included in logistic regression analysis to evaluate factors independently associated with PF. An ROC curve was utilized to determine the best predictive performance between DFA1 and PF.
RESULTS:
DFA1 was recorded in 536 of 2724 patients who underwent PR, including patients undergoing pancreaticoduodenectomy (n=380), distal pancreatectomy (n=140), and enucleation (n=16). PF occurred in 92 subjects (17.2%). On univariate analysis, DFA1, increased body mass index (BMI), small pancreatic duct size and soft pancreatic texture were significantly associated with PF (p < 0.05). On multivariate analysis, all four of these factors were independently associated with PF (p<0.05). Among multiple DFA1 cutoff values, 60 U/L demonstrated the highest sensitivity (Se) and negative predictive value (NPV, Table 1). 141 (26.3%) subjects satisfied this DFA1 cutoff. ROC confirmed the significant relationship between DFA1 and PF, with a c-statistic of 0.761 (Figure 1).
CONCLUSIONS:
Although DFA1 >5000 U/L was associated with high specificity, the sensitivity was low, thus decreasing its clinical usefulness. Lower DFA1 levels resulted in improved NPV; for example, a cut-off of 100 would incur only 4% FN rate while allowing 31% (170/536) of patients to undergo early drain removal safely. Therefore, in patients with low DFA1, early drain removal is recommended. Table 1. PF rates associated with DFA1 cutoffs (p < 0.001). | DFA1 (U/L) | DFA1 total (n < DFA1) | PF (n) | PF (%) | Se (%) | Sp (%) | NPV (%) | PPV (%) | | 5000 | 382 | 36 | 23.4 | 39.1 | 85.8 | 87.2 | 36.7 | | 2500 | 351 | 55 | 29.7 | 59.8 | 79.1 | 90.5 | 37.2 | | 350 | 243 | 75 | 25.6 | 81.5 | 54.7 | 93.5 | 27.2 | | 100 | 170 | 85 | 23.2 | 92.4 | 38.3 | 96.0 | 23.7 | | 90 | 163 | 89 | 23.9 | 96.7 | 36.7 | 98.2 | 24.1 | | 60 | 141 | 90 | 22.7 | 97.8 | 31.8 | 98.6 | 22.9 | | 25 | 69 | 90 | 19.3 | 97.8 | 15.5 | 97.2 | 19.4 |
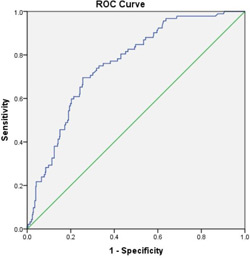 Figure 1. ROC curve (c-statistic = 0.761; p < 0.0001; CI 0.711 - 0.810).
Back to 2014 Annual Meeting Abstracts
|


